Case report
We report on the case of an 11-month-old boy with tuberous sclerosis with frequent ventricular arrhythmias secondary to cardiac rhabdomyomas. He was diagnosed at 1 week of age and had two cardiac arrests secondary to torsade de pointes in the paediatric ICU in his first couple of weeks of life. There were two large cardiac rhabdomyomas in the left ventricle, one in the apex and one in the outflow tract. He developed a long QT interval secondary to anti-arrhythmic medications, but was eventually controlled on a β-blocker and mexiletine. He was discharged and spent 8 months at home without any cardiac concerns. He was treated with gabapentin for seizures related to his tuberous sclerosis.
A Holter monitor was performed for routine monitoring as an outpatient and he was noted to have several fast runs of monomorphic ventricular tachycardia (Fig 1). This appeared to be around the time when he was having an episode of viral bronchiolitis. He was admitted for monitoring immediately after reviewing the Holter monitor; however, despite 3 days in hospital, no ventricular tachycardia was seen. We checked his mexiletine level, which was just below therapeutic range. Given that he was so difficult to control initially, and the limited experience with mexiletine in children, we were reluctant to increase the dose if these ventricular rhythms were now under control. We elected to place the new Reveal LINQ™ (Medtronic, Minneapolis, Minnesota, United States of America) that automatically alerts the implanting centre via Carelink if any alarm criteria are triggered. This would allow us to monitor him at home on a daily basis and look to change his anti-arrhythmic medications if ongoing ventricular tachycardia was seen .

Figure 1 Ventricular tachycardia seen on the 24 hour Holter monitor.

Figure 2 Carelink transmission showing demonstrating asymptomatic ventricular couplet, which was appropriately detected by the device.
The procedure was performed under general anaesthesia. Antibiotics were administered intravenously at the beginning of the case. Local anaesthetic was infiltrated around the incision site. A 1-cm cut was made in the skin and the device was inserted underneath the fascia in the left supra pectoral region (V2) using the insertion tool provided. This is where we saw the least artefact and T-wave oversensing. The skin was closed using a suture. The procedure was followed up with a chest X-ray, antibiotic cover, and interrogation of the device. He was discharged home the following day and has had only occasional couplets seen on his Carelink transmissions (Fig 2). He continues on the same dose of medications.
Discussion
The new Medtronic Reveal LINQ™ is now available at considerably more cost than the conventional Reveal™ loop recorder.Reference Bartoletti, Bocconcelli and De Santo 1 , Reference Krahn, Pickett and Sakaguchi 2 Its smaller size makes it much more suitable for children and infants (Fig 3). Although it is marketed as a device that can be implanted without general anaesthetic, this is usually not practical in children. It is technically easy to implant; however, slight modifications may be required to achieve optimal sensing of ventricular signals. It is best to implant using the provided insertion kit so as to create a tight pocket; however, certain modifications may be required. These include placing the device under the fascia, suturing it in place, or altering the position for better sensing. The device is marketed with the CareLink package, which is useful for remote monitoring of certain patients, either because of difficulty attending hospital appointments or if there is a high level of concern about the arrhythmia being monitored.Reference Cronin, Ching, Varma, Martin, Wilkoff and Lindsay 3 One of the advantages of the Reveal LINQ™ over the conventional loop recorder is an automatic transmission to CareLink should any alarm criteria be triggered. This makes the device ideal for patients who may be asymptomatic but require constant monitoring for dangerous tachycardias and bradycardias.
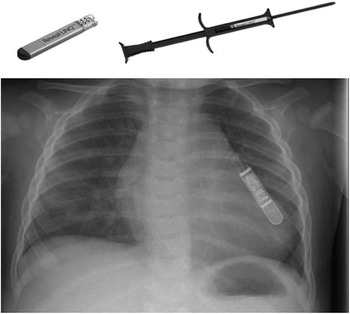
Figure 3 Medtronic LINQTM with insertion tool (top). Chest X-ray showing size of the device at the left sternal border (below).
There are currently no long-term data as to the efficacy of this device. Some of the potential difficulties in the future may be removing the device owing to its smaller size. It is unclear whether the device can be left in the patient long term. For patients who want to avoid a scar on their chest, it has always been our preference to implant the conventional loop recorder in the axillary area. It is unknown whether this is possible using the Reveal LINQ™. The increased cost of the device means that its use may be limited to selected patients who fulfil the above criteria.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.






