Hyperuricaemia, the cardinal biochemical feature and prerequisite for gout, is classified as primary (idiopathic/genetic) or secondary (acquired) and both may in turn classified into three functional types depending on whether there is an increased production of uric acid, a decreased excretion of uric acid, or a combination of both. However, in the past few decades overproduction has increased coinciding with a higher incidence of obesity and lifestyle factors, with the prevalence of hyperuricaemia reaching 18.0% in the Caucasian population.Reference Rho, Zhu and Choi 1 Moreover, hyperuricemia has become a cardiovascular risk marker associated with other classic cardiovascular risk factors, atherosclerosis and mortality.Reference Ford 2 , Reference Viazzi, Leoncini and Ratto 3
With the objective of gaining insight into the role of serum uric acid levels in congenital heart disease patients, we measured and compared serum uric acid levels with other demographic, clinical, and analytical parameters and determined survival curves between congenital heart disease patients with low and high serum uric acid concentrations.
Methods
Data were collected from all consecutive clinically stable congenital heart disease patients who had serum uric acid concentration measured in our Adolescent and Adult Congenital Heart Disease Unit of the Complejo Hospitalario Universitario Insular-Materno Infantil of Gran Canaria, between January, 2006 and January, 2013. Most of the patients included in the study, or their parents, gave informed consent for routine serum and 24-hour urine analytical determinations. In the remaining patients, serum uric acid concentrations were determined by review of the medical history. Repeated measurements, other than the first, were excluded from analysis. The inclusion criteria specified patients older than 14 years with a structural congenital heart disease. On the contrary, exclusion criteria were patients who had, at the time of analytical extraction, advanced kidney disease, were under high doses of diuretic treatment or chemotherapeutic agents, or did not give prior authorisation for analytical extraction. Measurements made after emergency admissions or within 6 months after surgery were also excluded from the analysis. Clinical data were acquired from patient records; specific congenital heart disease diagnoses were previously verified by echocardiography, cardiovascular magnetic resonance, and/or cardiac catheterisation. Patients were classified into diagnostic groups according to the underlying cardiac anatomy. Patients with more than one defect were classified according to the prevalent lesion from a clinical and/or haemodynamic point of view. An additional clinical subgroup of congenital heart disease patients was created according to whether or not the patient had hypoxaemia and patients were classified as hypoxaemic when basal oxygen haemoglobin saturation was ≤92%. Hyperuricaemia was established as a serum uric acid level >7.0 mg/dl in men and >5.7 mg/dl in women. 4 Serum creatinine, glucose, C-reactive protein, N-terminal-pro-B-type natriuretic peptide, and 24-hour urine uric acid concentrations were also recorded, when available, on the same date. Missing values were <10% for all variables. All the patients were Caucasian, and the protocol of the study was approved by the hospital's ethics committee. Survival status and time to death were obtained from the National Health Service computer system or after reviewing the medical history of the patients who died at the hospital.
Body weight and height were measured with the patients wearing light clothes and barefoot. Body mass index was determined according to the equation: weight/height2 in kg/m2. Blood samples were collected for subsequent laboratory analysis after an overnight fast of at least 10 hours, and during the 24-hour urine collection patients were recommended to have the usual diet and drink fluids, avoiding drinking alcohol or doing exercise strenuously before and during the urine collection. Analytical determinations were obtained for serum uric acid (normal values: 2.6–6.0 mg/dl), total cholesterol (20–220 mg/dl), low-density lipoprotein-cholesterol (0–155 mg/dl), high-density lipoprotein-cholesterol (45–75 mg/dl), triglycerides (30–200 mg/dl), C-reactive protein (0–0.5 mg/dl), N-terminal-pro-B-type natriuretic peptide (0–125 pg/ml), and uric acid in 24-hour urine (250–750 mg/24 hours) concentrations. Serum creatinine, uric acid, lipids, C-reactive protein, and 24-hour proteinuria were measured by spectrophotometry with an Olympus AU 2700 equipment (Olympus Diagnostic, Hamburg, Germany), and N-terminal-pro-B-type natriuretic peptide was measured by immunoassay using the Siemens Stratus CS Acute Care Diagnostic System (Siemens Healthcare Diagnostics Inc., Newark, Deusa, United States of America). The low-density lipoprotein-cholesterol (in mg/dl) was determined with the Friedewald formula (low-density lipoprotein = total cholesterol – [high-density lipoprotein+triglycerides/5]). Pulmonary arterial hypertension was defined as an increase in mean pulmonary arterial pressure greater or equal to 25 mmHg at rest as assessed by right heart catheterisation or echocardiography. Total mortality was defined as death from any cause, including cardiovascular.
Quantitative variables were expressed as mean ± standard deviation or median and 5th and 95th (5; 95) percentiles. Qualitative variables were expressed as percentages. Possible associations between categorical variables were evaluated using the Pearson χ2 test or the Student's t-test for continuous data. The non-parametric Mann–Whitney U test was used to compare two independent samples when the assumption of normality or homogeneity of variance was not met. Binary logistic regression multivariate analysis was performed to compare male congenital heart disease patients with serum uric acid concentrations ≤7.0 mg/dl and >7.0 mg/dl and female congenital heart disease patients with serum uric acid concentrations ≤5.7 mg/dl and >5.7 with those independent variables that had a p-value inferior to 0.05 in the univariate analysis. The results were expressed as odds ratios with their 95% confidence intervals. The log-rank test was used to compare Kaplan–Meier survival curves. Data analysis was carried out using SPSS 20.0 (SPSS, Chicago, Illinios, United States of America).
Results
From a total of 528 congenital heart disease patients followed up in our unit, 329 patients, including 190 male and 139 female patients, fulfilled the inclusion criteria. Overall, there were 39 hypoxaemic congenital heart disease patients, of whom 37 patients had uric acid determination.
Table 1 shows the different types of congenital heart diseases and the number and type of congenital abnormalities that had associated hypoxaemia and pulmonary artery hypertension. The prevalence, according to the NHANES III laboratory definition, of hyperuricaemia was 30 (15.8%) patients among men and 30 (21.6%) patients among women. Table 2 summarises the demographic characteristics, the clinical data, and the laboratory test results of male congenital heart disease patients with serum uric acid levels of 7.0 mg/dl or lower and those with levels exceeding 7.0 mIU/L. Table 3 summarises the demographic and clinical data and laboratory test results of the female congenital heart disease patients with serum uric acid levels of 5.7 mg/dl or lower and those above 5.7 mIU/L. Meanwhile, when compared with non-hypoxaemic congenital heart disease patients hypoxaemic patients had significantly higher serum uric acid levels (6.3 (4.1; 12.1) versus 5.2 (3.4; 7.9) mg/dl, p < 0.001) and significantly lower 24-hour urine uric acid concentrations (397.2 (79.0; 759.1) versus 486.6 (209.7; 895.7) mg/24 hours, p = 0.006). Similarly, comparing men and women, men had higher serum uric acid levels (5.8 (3.9; 8.4) versus 4.6 (3.2; 8.8) mg/dl, p < 0.001) and higher urine 24-hour uric acid concentrations (528.0 (216.8; 1038.6) versus 395.9 (118.7; 733.1) mg/24 hours, p < 0.001) than women. Moreover, only one patient with hyperuricaemia reported having gout and being under allopurinol treatment.
Table 1 Types of congenital heart diseases and congenital heart disease patients with and without hypoxaemia.
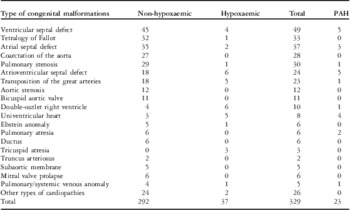
PAH = Pulmonary arterial hypertension
Patients with atrioventricular septal defect included 13 patients with partial and 11 patients with complete defect. Patients with transposition of the great arteries included 12 patients with dextro-transposition and 11 patients with levo-transposition of the great arteries
Table 2 Demographic data and laboratory test results from Congenital Heart Disease (CHD) male patients.

BMI = body mass index; CRP = C-reactive protein; HDL = high-density lipoprotein; LDL = low-density lipoprotein; NT-pro BNP = N-terminal-pro-B-type natriuretic peptide; PAH = Pulmonary arterial hypertension
Quantitative variables are expressed as mean ± standard deviation or median and 5th and 95th (5; 95) percentiles; qualitative variables are expressed as percentages of total
Table 3 Demographic data and laboratory test results from congenital heart disease female patients.

BMI = body mass index; CRP = C-reactive protein; HDL = high-density lipoprotein; LDL = low-density lipoprotein; NT-pro BNP = N-terminal-pro-B-type natriuretic peptide; PAH = Pulmonary arterial hypertension
Quantitative variables are expressed as mean ± standard deviation or median and 5th and 95th (5; 95) percentiles; qualitative variables are expressed as percentages of total
*One death of cardiac origin and two deaths of non-cardiac origin
**All deaths were of cardiac origin
In the binary logistic regression multivariate analysis performed to compare serum uric acid concentrations in male congenital heart disease patients with serum uric acid levels ≤7 mg/dl or >7 mg/dl, being hypoxaemic (odds ratios, 14.2; 95% confidence intervals, 3.7–55.2; p < 0,001) and having higher body mass index (1.14 (1.04–1.26); p = 0.007), higher serum creatinine (86.6; 3.2–3231.7, p = 0.008), and higher low-density lipoprotein-cholesterol (1.02; 1.002–1.04, p = 0.026) levels proved to be a risk factor for hyperuricaemia. On the contrary, in the binary logistic regression multivariate analysis performed to compare serum uric acid concentrations in female congenital heart disease patients, age (1.05; 1.002; 1.09, p = 0.042) and C-reactive protein concentrations (7.9; 2.3–26.7, p = 0.001) proved to be risk factors for hyperuricaemia.
During a median follow-up of 90.8 (4.5; 173.9) months, 16 out of 528 congenital heart disease patients died – 14 patients of cardiac origin and two patients of non-cardiac origin. In all of them, except in one male patient who died of cardiac cause, serum uric acid concentrations were determined. In all, 10 hypoxaemic congenital heart disease patients died in the follow-up, of whom five had pulmonary arterial hypertension. Overall, the highest mortality was observed between patients with atrioventricular septal defect (three patients) and double-outlet right ventricle (three patients), of whom two and one patient, respectively, had pulmonary arterial hypertension. The mean age from birth to serum uric acid determination was 29.2 ± 14.3 years. The median time to death was 50.2 (2.5; 123.5) months, and median age at death was 31.1 (15.0; 63.6) years. Kaplan–Meier survival analysis showed no significant differences between male congenital heart disease patients with low and high serum uric acid concentrations (≤7.0 and >7.0 mg/dl) (p = 0.633). However, it showed differences between hypoxaemic and non-hypoxaemic male congenital heart disease patients (p = 0.045) and almost significant differences between male patients with and without pulmonary artery hypertension (p = 0.053). No significant differences were seen between female congenital heart disease patients with high and low (≤5.7 and >5.7 mg/dl) serum uric acid concentrations (p = 0.189), between hypoxaemic and non-hypoxaemic female patients (p = 0.768), and between female patients with and without pulmonary artery hypertension (p = 0.364).
Discussion
Previous reports indicate that serum uric acid levels increase with ageReference Rho, Zhu and Choi 1 and that the difference in serum uric acid levels between genders could be in relation to an increased renal urate clearance by oestrogen in women, particularly before menopause.Reference Nicholls, Snaith and Scott 5 However, the serum uric acid levels have increased in parallel, over the past few decades, in both men and women probably in relation to an increasing burden of obesity, metabolic syndrome, lifestyle factors such as alcohol consumption, medications that increase uric acid concentrations such as aspirin or diuretics, and dietary habits.Reference Rho, Zhu and Choi 1 In fact, incorporating massively fructose in the diet in relation to sugar added to foods or sugar-sweetened drinksReference Gao, Qi and Qiao 6 seems to favour obesity and hyperuricaemia in relation to a decreased renal clearance of uric acid, an increased rate of purine synthesis de novo, and an accelerated degradation of purine ribonucleotides.Reference Fox and Kelley 7
In fact, multiple studies have associated hyperuricaemia with obesity and the metabolic syndrome in adults,Reference Cohen, Krause and Fraser 8 adolescents,Reference Ford, Li and Cook 9 and children,Reference Invitti, Maffeis and Gilardini 10 linking hyperuricaemia among younger adults with the risk of subsequent hypertension, insulin resistance, diabetes, and dyslipidaemia.Reference Feig, Kang and Johnson 11 In our study, the average body weights of the hyperuricaemic patients were greater than those of non-hyperuricaemic patients, which may explain the higher levels of serum triglyceride seen in the hyperuricaemic group. Moreover, those male congenital heart disease patients with high serum uric acid concentrations also had significantly higher total cholesterol and low-density lipoprotein-cholesterol levels than those male congenital heart disease patients with lower serum uric acid concentrations, which may also explain the higher incidence of metabolic syndrome seen in hyperuricaemic patients. However, no significant differences were seen in serum glucose concentrations between male and female congenital heart disease patients with high and low serum uric acid concentrations.
In addition, we found, both men and women, higher serum creatinine levels among the hyperuricaemic patients. Owing to the fact that ∼70% of uric acid is excreted from the kidney, abnormal renal haemodynamics, commonly seen in the initial stages of the disease, account for the increased serum urate concentration. However, hyperuricaemia seen in such renal diseases may also play a role in the progression of renal disease inducing renal arteriolopathy and the development of renal failure.Reference Ho, Tsai and Yu 12 On the other hand, hypoxaemia in congenital heart disease patients could be in relation to renal impairment, diuretic treatment, and renal hypoperfusion, which increases renal uric acid reabsorption.Reference Rho, Zhu and Choi 1 , Reference Hayabuchi, Matsuoka and Akita 13 , Reference Martínez-Quintana, Rodríguez-González and Fábregas-Brouard 14
In relation to C-reactive protein concentrations, increases in C-reactive protein levels are known to be a risk factor not only for cardiovascular diseases, but also for renal dysfunction.Reference Ruggiero, Cherubini and Ble 15 , Reference Nakagawa, Kang and Feig 16 Moreover, uric acid has been shown to directly stimulate the production of inflammatory mediators, such as C-reactive protein, in vascular cells, which is why it has been suggested that uric acid is an endothelium-injuring factor.Reference Kang, Park and Lee 17 Thus, these results imply that uric acid induces endothelial dysfunction and vascular inflammation reaction, which play pivotal roles in the pathogenesis of atherosclerosis.Reference Libby, Ridker and Maseri 18
With regard to morbi-mortality, data in the literature indicate that men with serum uric acid concentration above 6.7 mg/dl have a significantly higher incidence of congestive heart failure and stroke mortality than men with serum uric acid concentration below 4.6 mg/dl.Reference Strasak, Ruttmann and Brant 19 Similarly, women over 50 years of age and a serum uric acid level above 5.4 mg/dl have an increased mortality risk of coronary heart disease, congestive heart failure, and stroke when compared with women under 3.7 mg/dl.Reference Strasak, Kelleher and Brant 20 , Reference Ioachimescu, Brennan and Hoar 21 However, although most studies support the association between serum urate levels and cardiovascular mortality, some authors have reported negative results.Reference Baker, Krishnan and Chen 22 In this context, Krishnan et alReference Krishnan and Sokolove 23 found no association between hyperuricaemia and cardiovascular mortality when comparing middle-aged patients with and without hyperuricaemia. Similarly, Culleton et alReference Culleton, Larson and Kannel 24 found no significant associations in men and women after adjustment for cardiovascular risk factors and diuretic treatment, raising the question whether hyperuricaemia and cardiovascular disease and cardiovascular death is confounded by other factors in the cardiovascular disease causal pathway.
For this reason, it should be noted that hyperuricaemia itself does not appear to be a risk factor as commonly believed, despite serum uric acid stimulating oxidative stress, endothelial dysfunction, inflammation, and vasoconstriction, but a risk marker signalling the risk for the development of additional clinical complications such as coronary artery disease, renal failure, or hypertension.Reference Culleton, Larson and Kannel 24 , Reference Grassi, Ferri and Desideri 25 In our series, no significant differences were seen in survival, probably because it was a young population, with little or no associated cardiovascular risk factors and no previous history of cardiovascular events.
Although asymptomatic secondary hyperuricaemia is not an indication for routine therapy, we should look to those patients with hyperuricaemia to avoid overweight and obesity and a sedentary lifestyle. This is particularly true in hypoxaemic congenital heart disease patients because their hearts make them more vulnerable to adverse cardiac events, and in patients with coarctation of the aorta because they have a significant prevalence of hypertension by adolescence, sometimes despite surgical or percutaneous correction, leading to a subsequent risk of early morbidity and death.Reference Kenny and Hijazi 26
Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.





