Neonates with CHD who undergo cardiac surgery on cardiopulmonary bypass are at high risk for major morbidity and mortality post-operatively. According to the Society for Thoracic Surgery Congenital Heart Surgery Database from 2014 to 2018, neonates had an operative mortality rate of 8.1% compared to rates of 2.7 and 1.0% in their infant and child counterparts, respectively.1
Several neonatal-specific risk factors for adverse outcomes following cardiac surgery have been identified, such as gestational age, low birth weight, the complexity of the surgical procedure, and single ventricle physiology.Reference Costello, Pasquali and Jacobs2–Reference Kansy, Tobota, Maruszewski and Maruszewski5 A pre-operative model to predict risk of mortality in neonates undergoing cardiac surgery has also previously been developed. The model is composed of four variables, including two-ventricle versus single ventricle surgery, Apgar score, coexisting genetic syndrome, and age at pre-surgical hospital admission. This model was found to predict risk of 35-day post-operative mortality with an area under the curve of 0.80.Reference Clancy, McGaurn and Wernovsky6 The predominant focus of these prior investigations has been on pre- and intra-operative factors rather than on the post-operative pathophysiology that precedes major adverse outcomes and death.
Lactate is a by-product of anaerobic metabolism and thus is a potential surrogate marker of inadequate tissue oxygenation. Consequently, lactate has been evaluated as a biomarker to risk stratify patients who undergo cardiac surgery. In particular, hyperlactataemia in neonates following cardiac surgery has been shown to be associated with and predictive of a higher risk for major complications and mortality. However, small patient numbers, the variable timing of lactate measurements, and different outcome measures make definitive interpretation of other published studies problematic.Reference Schumacher, Reichel and Vlasic7–Reference Cheung, Chui and Joffe10 Hence, the clinical applicability of lactate as a risk assessment tool for neonates post-cardiac surgery has yet to be clearly delineated.
In this study, we aim to evaluate the ability of hyperlactataemia to primarily predict acute post-operative complications and mortality and to secondarily indicate the presence of clinically significant residual lesions in a large cohort of neonates following cardiac surgical repair or palliation. We hypothesise that post-operative hyperlactataemia will have clinical utility in the prediction of adverse outcomes post-operatively following neonatal cardiac surgery.
Materials and methods
Study design
Following approval by the institutional review board at Boston Children’s Hospital, a retrospective, observational study was performed in neonates (age ≤ 30 days) with CHD who underwent surgical correction or palliation on cardiopulmonary bypass from June 2015 through June 2019.
The primary explanatory variable was blood lactate levels (mmol/l) at defined time points and intervals, as follows: the value on admission to the cardiac ICU following transfer from the operating room; the delta level from cardiac ICU admission to 12 hours; and the peak value within the initial 48 post-operative hours in the cardiac ICU or earlier if the defined outcome occurred prior to 48 hours. Blood lactate levels are routinely collected on cardiac ICU admission and frequently thereafter (i.e. 12, 24, 48 hours, and more often if clinically indicated) during the acute post-cardiac surgery period, allowing for it to be studied accordingly. The covariates included pre-operative patient characteristics (age, gender, gestational age), surgical complexity as defined by the Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery mortality category, the need for emergent surgery, and single ventricle physiology. The patients were specifically stratified into two risk categories, Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery categories 1, 2, and 3 versus Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery categories 4 and 5, given the small number of neonates who underwent surgeries classified as Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery 1, 2, or 3.
The primary endpoint was a composite outcome, defined as having one or more of the following adverse events: need for extracorporeal membrane oxygenation support or cardiac arrest within 72 hours or in-hospital mortality within 30 days post-operatively. The secondary outcome was the adequacy of surgical repair as measured by the technical performance score. The technical performance score is a tool that was developed to grade the adequacy of surgical repair as ‘Class 1: trivial or no residua, optimal; Class 2: minor residua, adequate; or Class 3: major residua or reintervention for major residua during index hospitalisation, inadequate’ based on echocardiographic and clinical criteria.Reference Larrazabal, del Nido and Jenkins11,Reference Nathan and Karamichalis12
Data collection
The institutional electronic database was queried for all neonatal cardiac surgery encounters. In addition to the aforementioned explanatory variable and covariates, additional patient demographics and intraoperative data were collected. Patients were excluded if any of these variables were missing or if they were cannulated to extracorporeal membrane oxygenation intraoperatively prior to cardiac ICU admission. If a patient had multiple surgical encounters, only the initial surgery was included. The components of the composite outcome were collected via review of the electronic medical record and verified by two members of the research team.
Statistical analysis
Demographics, patient characteristics, and outcomes were presented as a median (interquartile range) for continuous variables and as a frequency (%) for categorical variables. The Wilcoxon rank sum test was applied to compare lactate levels between those who had the outcome versus those who did not. Univariate and multi-variable logistic regressions were utilised to evaluate the association between the primary explanatory variable and covariates with the composite outcome. Statistically significant variables in univariate modelling with p < 0.05 were included in the multi-variable analyses. Due to collinearity between lactate measures, each lactate variable was examined in separate multi-variable models to determine if each was an independent predictor of the composite outcome after adjusting for significant baseline factors and characteristics. The results are displayed as odds ratios with 95% CI. To analyse the discriminatory ability of the lactate measures that were found to be significant independent predictors of the composite outcome from multi-variable modelling, receiver operating characteristic curve analysis was used and the area under the receiver operating characteristic curve was obtained. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated at various lactate thresholds. Positive predictive value and negative predictive value were calculated using Bayes’ formula in order to incorporate the prevalence of the composite outcome. Youden’s J index was used to determine the optimal cut-points to maximise the sum of sensitivity and specificity. A two-sided α level of 0.05 was used to determine statistical significance.
The significant independent predictors identified in the multi-variable logistic regression were included in a risk model. Continuous variables were dichotomised according to their optimal cut-points based on Youden’s J index. Predicted probabilities of the outcome were calculated for each covariate pattern with a 95% CI. Model performance of the multi-variable risk score was evaluated by the area under receiver operating characteristic curve as measure of discrimination and by the concordance calibration coefficient and the Brier score to evaluate calibration. An area under the curve of at least 0.70 was considered to demonstrate good model discrimination.Reference Hosmer, Lemeshow and Sturdivant13 An internal bootstrap model validation was performed with 1000 bootstrap resamples to evaluate the internal validity of the multi-variable risk model.Reference Altman, Vergouwe, Royston and Moons14
As a secondary analysis, the associations between lactate and discharge technical performance score were examined using logistic regression modelling and receiver operating characteristic analysis.
Statistical analysis was performed using Stata (version 16.0; StataCorp, College Station, TX, USA).
Results
A total of 470 patients were reviewed. Thirty-eight patients were excluded due to missing technical performance score data related to incomplete echocardiographic data; 432 neonates were ultimately included in the analysis. Patient demographics and other characteristics are displayed in Table 1. The incidence of the composite outcome was 6.5% (28/432). Forty-six percent of the adverse events occurred within 12 hours of admission to the cardiac ICU post-operatively (Table 2). The post-operative time to composite outcome is the time to the first adverse event in patients who had more than one adverse event.
Table 1. Demographics and patient characteristics

Data are presented for the number of cases with non-missing observations for each variable
STAT = Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery; TPS = technical performance score
Table 2. Post-cardiac surgery adverse outcomes

ECMO = extracorporeal membrane oxygenation; TPS = technical performance score
* The composite outcome includes patients who had one or more of any of the three outcomes: cardiac arrest or extracorporeal membrane oxygenation within 72 hours or in-hospital mortality within 30 days post-cardiac surgery
Neonates who sustained the composite outcome had significantly higher lactate levels on cardiac ICU admission and peak lactate levels within 48 hours compared to those without the outcome with medians of 6 mmol/l (interquartile range: 3.9, 7.8) versus 4.1 mmol/l (interquartile range: 2.9, 6.3) (p = 0.006) and 6.1 mmol/l (interquartile range: 4.6, 10.6) versus 4.6 mmol/l (interquartile range 3.3, 6.4), respectively (p = 0.004). In patients with the outcome, the peak lactate was measured at a median of 2.9 hours (interquartile range: 1, 35) prior to the adverse event. Those with the outcome had an increase in median lactate level (0.4 mmol/l; interquartile range: −2.1, 3) from cardiac ICU arrival to 12 hours compared to those without the outcome who had a decrease in median level (−0.8 mmol/l; interquartile range: −2, 0.2) (p= 0.005) (Fig 1).
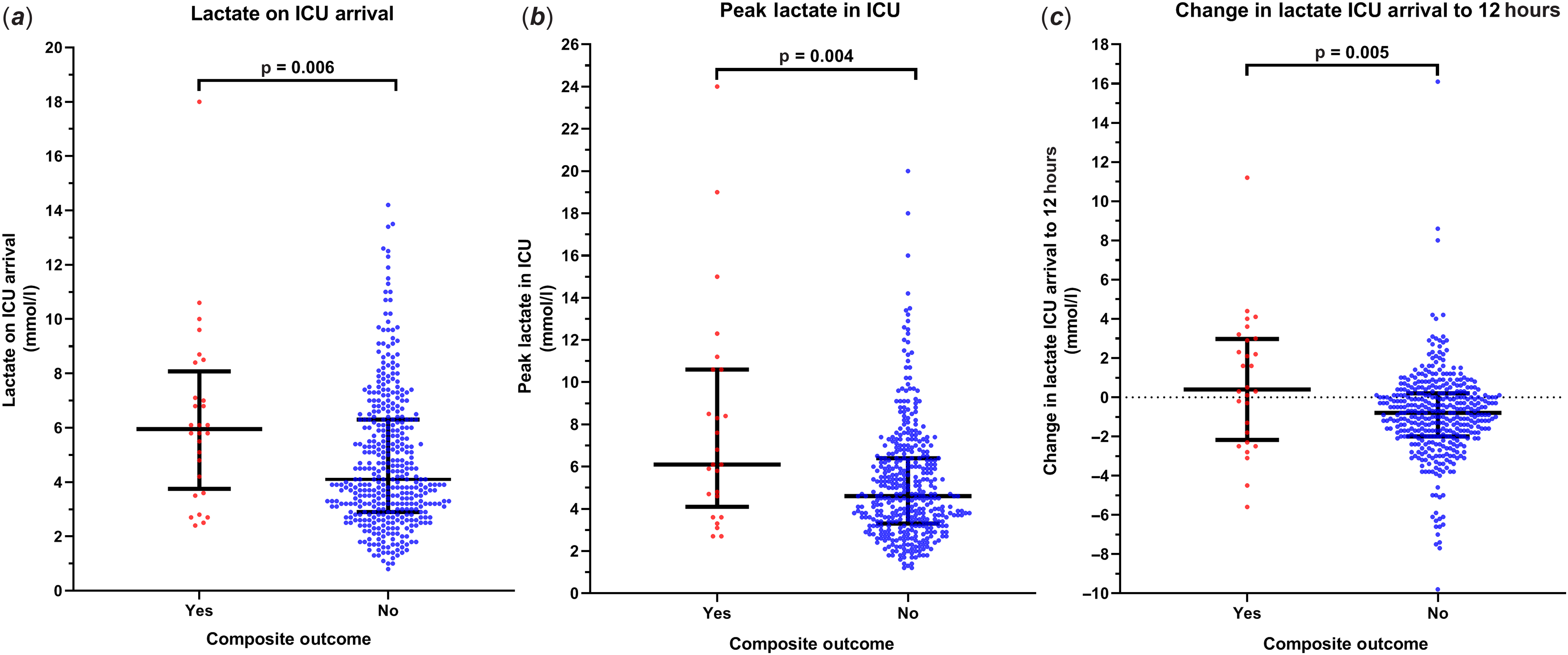
Figure 1. The distribution of blood lactate levels* according to the composite outcome**. (a ) The lactate level on post-operative admission to the cardiac ICU. (b) The peak lactate level within 48 hours post-operatively, or prior to the outcome if it occurred prior to 48 hours. (c) The change in lactate level from cardiac ICU admission to 12 hours post-operatively. *The lactate level is expressed as a median value with an interquartile range; **The composite outcome includes patients who had one or more of any of the three outcomes: cardiac arrest or extracorporeal membrane oxygenation within 72 hours or in-hospital mortality within 30 days post-cardiac surgery.
The lactate level on cardiac ICU arrival (odds ratios: 1.21; 95% CI: 1.07, 1.37), the peak lactate value (odds ratios: 1.22; 95% CI: 1.11, 1.35), and the delta lactate level from cardiac ICU admission to 12 hours (odds ratios: 1.26; 95% CI: 1.09, 1.45) were significantly associated with the composite outcome on univariate analysis. Single ventricle physiology (odds ratios: 4.16; 95% CI: 1.87, 9.28) was also statistically significant. The remaining covariates, including prematurity, weight, Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery category, and the need for emergent surgery, did not reach statistical significance (Table 3).
Table 3. Univariate logistic regression analysis of the composite outcome**

OR = odds ratio; CI = confidence interval; STAT = Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery; TPS = technical performance score
* Statistically significant
** The composite outcome includes patients who had one or more of any of the three outcomes: cardiac arrest or extracorporeal membrane oxygenation within 72 hours or in-hospital mortality within 30 days post-cardiac surgery
On multi-variable analysis while adjusting for single ventricle physiology, an increase in lactate level from cardiac ICU admission to 12 hours was a significant independent predictor of the composite outcome (odds ratios: 1.28 per mmol/l; 95% CI: 1.11, 1.49). Single ventricle physiology (odds ratios: 4.92; 95% CI: 2.15, 11.29) was independently associated with the outcome with adjustment for lactate measures.
On receiver operating characteristic analysis, delta lactate level had fair discrimination regarding the composite outcome with an area under the curve of 0.658 (95% CI: 0.522, 0.794). Per Youden’s J index, a cut-point for a change in lactate ≥1.6 mmol/l maximised sensitivity (46%) and specificity (91%). The positive predictive value and the negative predictive value were 26 and 96%, respectively.
A risk model for the composite outcome based on delta lactate from ICU arrival to 12 hours ≥1.6 mmol/l and single ventricle physiology is presented in Table 4. The evaluation of performance of the risk score revealed good discrimination (area under the curve = 0.792; 95% CI: 0.701, 0.882) and calibration (concordance calibration coefficient = 0.95; Brier score = 0.07). Internal bootstrap model validation of the risk score using 1000 bootstrap resamples determined a bias-corrected Somers’ D rank correlation of 0.64 and Nagelkerke R2 of 0.30. The bias-corrected intercept and slope of the logistic calibration equation were 0.03 and 1.01, respectively. Therefore, the bootstrap resampling results indicate strong internal validity of the predictive multi-variable risk score. As demonstrated in Table 4, there was a predicted probability of 1.8% (95% CI: 0.9–3.9%) when neither risk factor is present, and there was a predicted probability for the composite outcome of 52.4% (95% CI: 32.4–71.7%) when single ventricle physiology and delta lactate ≥1.6 mmol/l are observed.
Table 4. Predicted probability of the composite outcome*

* The composite outcome includes patients who had one or more of any of the three outcomes: cardiac arrest or extracorporeal membrane oxygenation within 72 hours or in-hospital mortality within 30 days post-cardiac surgery
In a secondary analysis of lactate measurements versus discharge technical performance score, 20% (80/432) of neonates were found to have a discharge technical performance score of 3. Peak lactate was significantly independently associated with discharge technical performance score of 3 (odds ratios per mmol/l: 1.27; 95% CI: 1.14, 1.42), while adjusting for other lactate measures and single ventricle physiology. Median and interquartile ranges for peak lactate by discharge technical performance score are displayed in Figure 2. From receiver operating characteristic analysis, it was found that a peak lactate ≥7.3 mmol/l maximised the combination of sensitivity (45%) and specificity (86%) in predicted discharge technical performance score of 3. This cut-off was associated with 5.16 times the odds of discharge technical performance score of 3 (odds ratios: 5.16; 95% CI: 3.01, 8.87).

Figure 2. The distribution of peak lactate level within 48 hours post-operatively by discharge TPS. Patients with discharge TPS of 3 had significantly higher peak lactate values than patients with discharge TPS of 1 or 2.
Discussion
The present study of neonates with CHD who undergo cardiac surgery demonstrates that the combination of the immediate post-operative trend in blood lactate and the single ventricle physiologic status provides a strong predictive model to identify those that are at high risk for early extracorporeal membrane oxygenation and cardiac arrest and 30-day mortality. In addition, peak blood lactate in the early post-cardiac surgery period is associated with significant residual lesions that may ultimately require intervention.
Blood lactate offers several advantages in the prediction of adverse outcomes in neonates following surgery for CHD. Hyperlactataemia is a consequence of the full range of pathophysiologic states that lead to major morbidity and mortality in this population, including decreased cardiac output, inadequate oxygen delivery, increased metabolic demand, and capillary leak syndrome. In addition, blood lactate is a practical laboratory test that is routinely available with results provided within the hour, allowing for anticipation of impending cardiovascular decompensation. While this study and others have demonstrated a significant association between hyperlactataemia and adverse outcomes in neonates who undergo cardiac surgery, use of lactate as a single predictor at the bedside is challenging. One such challenge is the lack of a consistent definition of hyperlactataemia. In addition, the post-operative time frames over which lactate has been analyzed differ among studies. In an analysis of 221 neonates who underwent the Norwood procedure with Sano modification, failure to clear lactate to <6.76 mmol/l within the initial 24 post-operative hours was discriminatory for 30-day mortality.Reference Murtuza, Wall and Reinhardt15 Time to normalisation of lactate levels of >48 hours was predictive of mortality among a small cohort of 22 neonates.Reference Kalyanaraman, DeCampli and Campbell16 Schumacher et al demonstrated that a rate of increase in lactate of ≥0.6 mmol/l per hour during the initial 24 post-operative hours was discriminatory for the need for extracorporeal membrane oxygenation, the need for dialysis, and mortality in a subgroup of 86 neonates with single ventricle and biventricular CHD.Reference Schumacher, Reichel and Vlasic7 These differences in study design preclude the ability to make direct comparisons of lactate as an independent predictor for clinical application.
In our study of a large cohort of neonates undergoing predominantly highly complex cardiac surgery, an absolute change in lactate ≥1.6 mmol/l from cardiac ICU admission to 12 hours was an independent predictor of early adverse events and 30-day mortality post-operatively. Greater than half of the adverse events occurred after 12 hours; therefore, the delta level from admission to 12 hours should permit adequate time for planning. However, 39.3% of patients experienced the adverse event within 6 hours of CICU admission, which is important to note given that haemodynamic decompensation, such as low cardiac output, can occur in the more immediate hours following cardiopulmonary bypass and cardiac surgery. Although the evaluation of lactate should occur at a frequency that is feasible for clinical practice, an earlier follow-up lactate level (e.g. 2–6 hours) can potentially improve the prediction of events that occur within the first 12 hours following CICU arrival.
Acknowledging the shortcomings of lactate as a single predictor, as well as the potential contribution of non-pathologic etiologies to hyperlactataemia, we included additional factors into the post-operative risk assessment of neonates with CHD (Table 3). Similar to prior studies, single ventricle physiology was found to be an independent predictor on univariate analysis of adverse outcomes and mortality post-cardiac surgery.Reference Kansy, Tobota, Maruszewski and Maruszewski5 It was also found to be a predictor in the multi-variable analysis along with a delta lactate ≥1.6 mmol/l from ICU arrival to 12 hours. The presence of single ventricle physiology and a 12-hour delta lactate of ≥1.6 mmol/l yielded a 52.4% probability of cardiac arrest, extracorporeal membrane oxygenation, or mortality, which is more than 6-fold and 2-fold the probability of either risk factor alone.
Interestingly, we did not find a significant association between higher risk procedures (as determined by Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery mortality categories) and the composite outcome. This finding may be explained by higher proportions of complex CHDs in our patient population, as indicated by the 72% of patients who were categorised as Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery 4 or 5 in our study. Of note, we elected to dichotomise the Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery category due to a limited number of composite events in order to fit a parsimonious multi-variable model. It is likely that physiologic compromise following surgical repair or palliation due to the presence of a substantial residual lesion or single ventricle status is a more important determinant of outcome than the technical complexity of the procedure itself. We did not include the duration of cardiopulmonary bypass as a variable in our analyses. Although cardiopulmonary bypass is known to induce systemic inflammation, which can lead to capillary leak and other hemodynamic derangements, several studies have revealed no association between cardiopulmonary bypass duration and mortality in neonates.Reference Schumacher, Reichel and Vlasic7,Reference Cheifetz, Kern, Schulman, Greeley, Ungerleider and Meliones9,Reference Kalyanaraman, DeCampli and Campbell16 In fact, it has been demonstrated that the time spent with mean arterial pressure < 25 mmHg during cardiopulmonary bypass is associated with increased lactate (per 30 min) (coefficient = 0.423; 95% CI 0.196–0.651; p < 0.001) or duration of circulatory arrest (per 30 min) (coefficient = 1.216; 95% CI 0.754–1.678; p < 0.001).Reference Nasr, Staffa and Boyle17
We specifically evaluated the technical performance score as a secondary outcome as several studies have demonstrated that suboptimal surgical repair is significantly associated with a greater severity of illness and a higher rate of mortality in neonates following cardiac surgery.Reference Nathan, Karamichalis and Liu18–Reference Mazwi, Brown and Marshall20 A peak lactate ≥7.3 mmol/l within 48 hours was found to be indicative of a discharge technical performance score of 3. Therefore, peak lactate levels can help to quantify the physiologic impact of residual lesion post-cardiac surgery and should prompt early echocardiography for possible surgical or catheter-based intervention.
Our study is limited by its retrospective study design. Notably, however, no patients were excluded for missing lactate data, therefore eliminating the bias that lactate was evaluated more frequently in neonates who were more critically ill and at higher risk. Although our data were obtained from a large referral centre for neonates with highly complex CHD, it must be acknowledged that this is a single institution dataset. Consequently, external validation is needed to generalise its applicability.
Conclusion
In summary, we present a discriminative simple and objective two-variable model to risk stratify neonates following cardiac surgical repair or palliation. This model highlights the impact of single ventricle physiology along with hyperlactataemia to distinguish at-risk neonates. In addition, peak lactate has been shown to be predictive of worse (Class 3) technical performance score and thus can guide earlier investigation and intervention on major residual lesions. The availability of a neonatal risk stratification tool could lead to improved outcomes via early identification and intervention of impending adverse events.
Acknowledgements
The authors thank the Information Technology team at the Department of Anesthesiology, Critical Care and Pain Medicine, the Department of Cardiac Surgery, and the Neonatal Cardiac Care Collaborative at Boston Children’s Hospital.
Financial support
This study was solely supported by the Department of Anesthesiology, Critical Care and Pain Medicine at Boston Children’s Hospital, Harvard Medical School, Boston, MA.
Conflict of interest
None.
Ethical Standards
This study was approved by the Institutional Review Board of Boston Children’s Hospital.









