In this report, we present a case of a 9-month-old girl who underwent division of a vascular ring through a left thoracotomy incision. On the 2nd day after surgery, she developed ipsilateral left-sided ptosis, miosis, and enophthalmos, which were not present before surgery. The ptosis was present at the time of her discharge from hospital.
This report reviews the available paediatric critical care literature and focuses on the possible surgically related aetiology, aiming to increase the awareness on this rare complication and to suggest possible strategies for avoidance.
Case presentation
The patient is a 9-month-old girl with congenital deafness, diminished visual acuity, and right-sided facial palsy for which she had regular paediatric follow-up. She also had a history of chronic stridor and was diagnosed to have a vascular ring in the form of a right-sided aortic arch with a retrooesophageal left subclavian artery and a small left-ductus arteriosus, patent ductus arteriosus. The patient was referred to our institute for elective cardiac surgery. After standard intubation and insertion of a left internal jugular vein central line in the operating room, she underwent vascular ring division, by dividing the patent ductus arteriosus, through a left posterolateral thoracotomy. She was transferred to the cardiac surgery ICU with a left-sided pleural tube.
She was extubated several hours after surgery and her post-operative course was uneventful except for the development of a mild chylothorax that required continued chest tube drainage for 4 days after surgery. On the 2nd post-operative day, the child was noted to have left-sided ptosis, miosis, and enophthalmos (Fig 1).
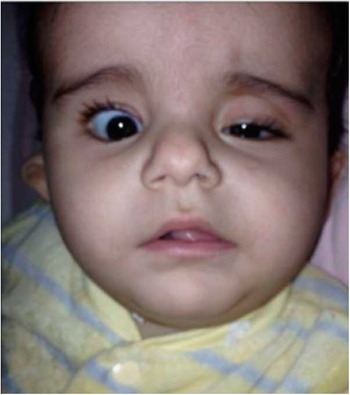
Figure 1 Patient post cardiac surgery.
With this classical triad of signs, she was diagnosed with Horner’s syndrome. The patient was treated expectantly and was discharged on post-operative day 7 with persistent findings of Horner’s syndrome.
Discussion
Horner’s syndrome was first described in 1869 by a Swiss ophthalmologist, Johann Friedrich Horner. Classically, it consists of the symptom triad of miosis, ptosis, and anhydrosis. It is caused by damage to or interruption of the oculosympathetic nerve pathway, somewhere between its origin in the hypothalamus and its destination in the eye.Reference Doğan, Erkoç, Sayarlıoğlu, Etlik and Uzun 1
There are many reported reasons for iatrogenic Horner’s syndrome. The most common causes seen in the ICU are related to trauma,Reference Starr, Shubert and Baumann 2 neck, or thyroid surgery,Reference Aslankurt, Aslan, Colak and Aksoy 3 thoracotomy tube placement,Reference Knyazer, Levy, Marcus and Lifshitz 4 thoracic surgery,Reference Kaya, Liman, Bir, Yuncu, Erbay and Unsal 5 and as a result of central venous catheter insertion.Reference Suominen, Levy, Marcus and Lifshitz 6
It has been postulated that a chest tube with a high insertion point may compress the stellate ganglion or post-ganglionic fibres, causing ischaemia and potential nerve injury because of neuropraxia of the second neuronal pathway. For that reason, it is usually recommended to keep the tip of the chest tube below the second intercostal space.Reference Kaya, Liman, Bir, Yuncu, Erbay and Unsal 5
Our patient had a well-fixed chest tube with acceptable position below the second intercostal space that, in our judgement, was unlikely to be in close direct contact with the stellate ganglion near the apex of the left lung. Nonetheless, a properly placed chest tube, even with radiologically satisfactory position, may still slide within the pleural cavity. Because of the small pleural cavity in infants and small children, the chest tube may retain enough mobility to reach the pleural apex, leading to direct ganglia or fibre contact and injury. This hypothesis could be a potential explanation for the development of Horner’s syndrome in our patient. In addition, the presence of an irritating foreign body such as a pleural tube may also induce insult, not only by direct compression, but also by local irritation and inflammation in the tissue adjacent to ganglia and/or post-ganglionic fibres, leading to the development of Horner’s syndrome.
Some authors have reported Horner’s syndrome following chest tube insertion with quick improvement of symptoms following removal of the offending chest drain. In one study, the authors reported complete resolution of chest tube-associated Horner’s syndrome following repositioning of chest drains away from the pleural apex.Reference Levy and Newman-Toker 7 , Reference Baird, Al-Balushi, Wackett and Bouchard 8
Horner’s syndrome does not always resolve after removal of a potentially offending pleural tube. In one published article, the authors reported a 3-month-old girl who developed persistent Horner’s syndrome after pleural tube insertion and despite subsequent pleural tube removal.Reference Bashkar, Lodha and Kabra 9 Another report documented a 3-year-old girl with Horner’s syndrome after chest tube insertion at the time of diaphragmatic hernia repair, with improvement documented 1-month post-operatively.Reference Ozel and Kazaz 10 In yet another report, the authors presented a 7-month-old girl with chest tube-associated Horner’s syndrome, with only partial improvement after 3 months of follow-up.Reference Knyazer, Levy, Rosenberg, Lifshitz and Lazar 11
Some authors emphasise that improvement may take several months following removal of the offending cause. Zagrodink et alReference Zagrodink and Kline 12 reviewed various reports on Horner’s syndrome following pleural tube placement and noted full resolution of the symptoms in only one-third of the cases. Furthermore, some patients may demonstrate partial improvement in some Horner’s syndrome symptoms, such as the resolution of anisocoria more than ptosis.Reference Knyazer, Levy, Marcus and Lifshitz 4 , Reference Baird, Al-Balushi, Wackett and Bouchard 8 Our patient’s symptoms, particularly ptosis, remained unchanged throughout the entire hospital course. Further follow-up is needed to determine whether future improvement occurs over time or whether permanent residual lesions persist. The presence of facial weakness in our patient before surgery may play a role in complicating recovery and could prolong the time of full resolution of symptoms. Nonetheless, we do not think there is a link between post-operative Horner’s syndrome and this patient’s pre-operative facial weakness that had been observed since birth.
Although uncommon, surgical dissection of the apical pleura is also reported to be associated with the development of Horner’s syndrome. Kaya et al reported two adult patients who developed Horner’s syndrome after surgical resection of apical lung cancer. They related the cause mainly to an apical pleural burn as a result of cauterisation during surgery.
Thoracic endovascular aortic repair applied for late aneurysm after coarctation repair has been reported to have high incidence of Horner’s syndrome, reaching 30.8% of patients needing this type of intervention.Reference Yazar, Budts, Maleux, Houthoofd, Daenens and Fourneau 13 Furthermore, Horner’s syndrome was also reported in patients needing subclavian flap aortoplasty for coarctation repair.Reference Naimer, Weinstein and Rosenthal 14 The division of a vascular ring, in our case report, was completed under direct vision with an open posterolateral thoracotomy incision. The surgical dissection near great arteries was reported to be smooth, limited, and uneventful, which make us consider that a post-ganglionic fibre injury due to dissection is unlikely, although it cannot be excluded entirely. There have been recent encouraging reports describing successful vascular ring division by a thoracoscopic approach.
Thoracoscopy is minimally invasive and requires less dissection. The experience with this promising technique is gradually developing with potential advantages for fewer complications in comparison with the traditional open surgical approach.Reference Koontz, Bhatia, Forbess and Wulkan 15 More studies are needed to establish the potential superiority of thoracoscopy in comparison with the traditional approach for the surgical management of vascular ring in infants and children.
Another possible iatrogenic cause for Horner’s syndrome that should be entertained is cervical central line insertion. Central venous catheter insertion in the internal jugular vein may injure the surrounding structures by causing a haematoma that can compress the sympathetic fibres. Direct damage to sympathetic fibres may also occur when the carotid artery is punctured accidentally during attempted cervical central venous line insertion or when multiple attempts are used during the insertion attempt.Reference Raja, Waheed, Aziz, Jamal and Khalid 16 – Reference Taskapan, Oymak, Dogukan and Utas 18
Furthermore, some authors suggested that extravasation of the fluid from a central line may generate a toxic effect on the ganglion and may induce direct injury leading to Horner’s syndrome.Reference Büyüktortop, Gürbüz-Köz, Atalay and Kural 19
Our patient had no complications during the left internal jugular central venous catheter insertion at the time of surgery. The insertion was recorded as being smooth and was not associated with the development of a haematoma or carotid artery puncture. Subsequent removal of the central line did not coincide with resolution of symptoms.
The majority of patients undergoing major cardiac surgery will require the placement of a central venous catheter and a pleural tube, both considered to be possible causes for iatrogenic Horner’s syndrome. With the implementation of ultrasound-guided device insertion, the risk for injury and complications during insertion is expected to be less and less over time. Further studies are needed to determine whether implementation of ultrasound-guided vascular access is associated with a decrease in such complications, particularly the incidence of Horner’s syndrome following central line insertion.
Conclusion
Horner’s syndrome in children after cardiac surgery is rare. The diagnosis of Horner’s syndrome immediately after cardiac surgery may not be easy, especially when the patient is sedated. A high index of suspicion with awareness of the possible causes is recommended. Avoidance of high chest tube insertion, implementation of ultrasound-guided central line insertion, and minimisation of surgical dissection in the area of the stellate ganglion may help minimise the potential causes of iatrogenic Horner’s syndrome in children undergoing cardiac surgery. Resolution of symptoms is variable and may be delayed.
Acknowledgements
I thank my committee for their continued support and encouragement. I also offer my sincere appreciation to Dr Kabbani who encouraged me in the completion of this project.
Financial Support
This report received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The institutional research committee approved the case report and family was consented and approved publication of their child photo.



