In congenital heart block associated with maternal anti-SSA antibody, especially after pacemaker implantation, cardiac dysfunction is not rare. Reference Moak, Barron and Hougen1–Reference Morel, Levesque and Maltret5 The aetiology of cardiac dysfunction in this setting is estimated to be pacing-induced or antibody-related myocardial injury. Reference Villain, Costedoat-Chalumeau and Marijon2,Reference Janousek, van Geldorp and Krupickova6 Although the effectiveness of cardiac resynchronisation therapy for pacing-induced cardiomyopathy has been reported, the prognosis of dilated cardiomyopathy related to autoimmune antibodies is poor. Reference Moak, Barron and Hougen1,Reference Villain, Costedoat-Chalumeau and Marijon2,Reference Morel, Levesque and Maltret5
Secundum atrial septal defect and arterial duct are known to be associated with cardiac lesions of congenital heart block; however, treatment for these lesions has scarcely been described. Reference Eronen, Siren and Ekblad4,Reference Buyon, Hiebert and Copel7 Herein, we report a case of a patient with maternal anti-SSA antibody-related congenital heart block accompanied by a large secundum atrial septal defect. She presented with cardiac dysfunction 4 years after pacemaker implantation, and endomyocardial biopsy was useful in deciding the course of treatment.
Case
A 4-year-old girl presented with oedema and shortness of breath. She was diagnosed with maternal anti-SSA antibody-related congenital heart block prenatally and with secundum atrial septal defect after birth. A temporary pacemaker was placed at 1 day, followed by a permanent pacemaker implant at 3 months old. The pacing mode was VVI, and the site was at the right ventricular outflow (epicardial lead). During follow-up, left ventricular diastolic dimension was small (22.4 mm at 2 years old, 24.4 mm at 3 years old), but the chest thoracic ratio, as measured on radiography, had gradually increased (56% at 2 years old, 67.9% at 3 years old). The left ventricular ejection fraction on echocardiography was approximately 50%, with evident ventricular desynchronisation. At 4 years, she was admitted to the hospital with eyelid oedema and fatigue. Auscultation revealed moist rales in the lung field and systolic ejection murmur at the third parasternal border. Chest radiography showed significant cardiomegaly, and electrocardiography showed a VVI pacing rhythm (110 bpm) with a complete left branch block pattern (Fig 1A, 1B). Brain natriuretic protein level was 3487 pg/mL. Echocardiography revealed dilated right chambers, severe tricuspid valve regurgitation, left ventricular ejection fraction of 32% with interventricular septum paradoxical motion, and a large secundum atrial septal defect (20 mm in diameter) (Fig 1C, 1D).
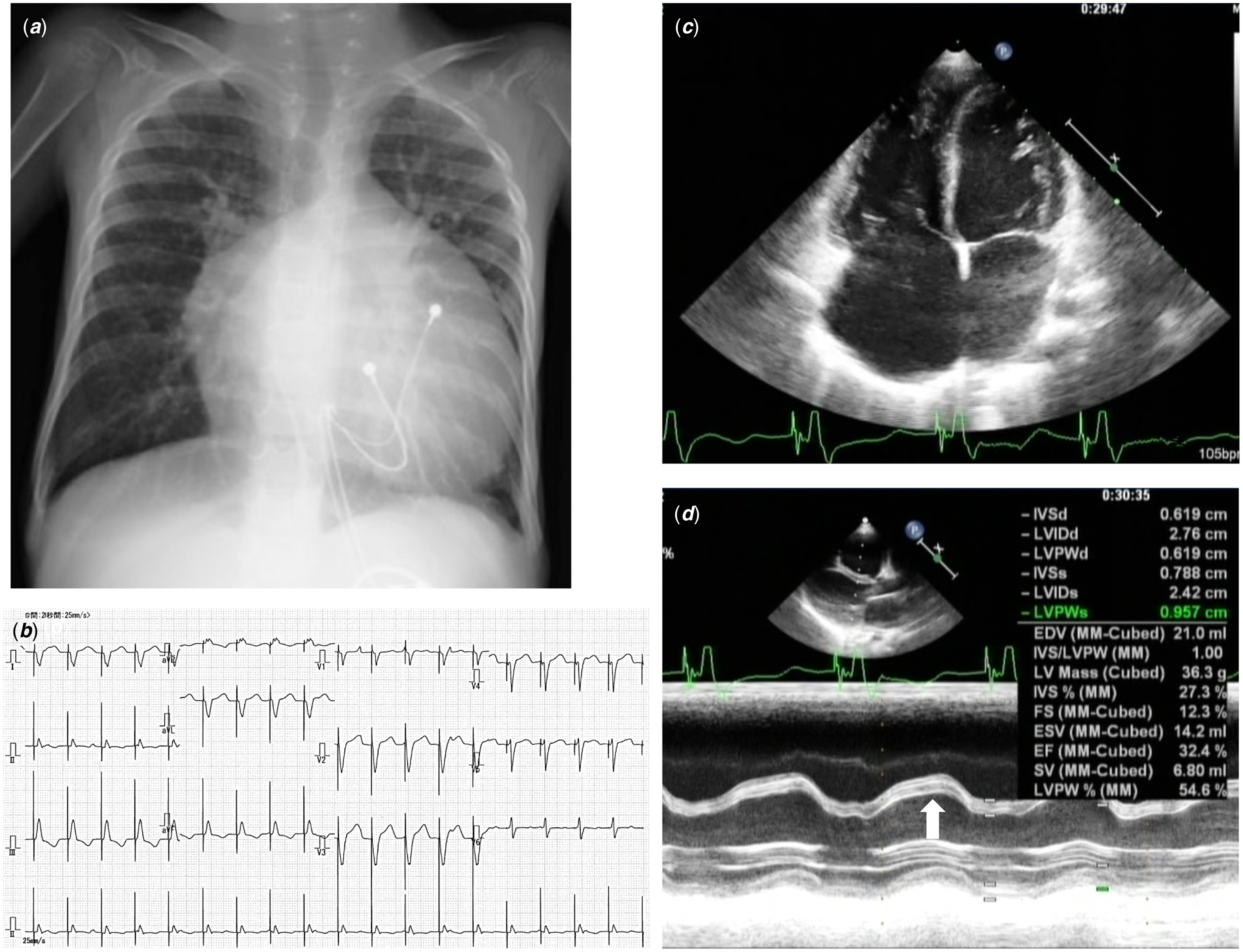
Figure. 1. ( a ) Chest X-ray image on admission showing marked cardiomegaly. ( b ) 12-lead electrocardiogram on admission. Pacing rhythm (VVI) with a heart rate of 110 bpm. QRS complex in V1 lead showing complete left bundle branch block pattern. ( c , d ) Echocardiogram on admission. ( c ) Four-chamber view showing dilated right atrium and right ventricle with large secundum atrial septal defect. ( d ) Left ventricular M-mode image demonstrated paradoxical septal motion (arrow).
The patient was treated with intravenous diuretics and olprinone. The brain natriuretic protein level decreased to 1972 pg/mL, and her clinical symptoms (oedema and fatigue) improved gradually; however, cardiomegaly persisted. Cardiac catheter examination was performed on the 15th day of admission, which revealed left ventricular dysfunction (ejection fraction, 42%; left ventricular end-diastolic pressure, 17 mmHg), mild pulmonary hypertension, and increased pulmonary blood flow (ratio of pulmonary blood flow to systemic blood flow 2.9). We speculated that the cause of cardiac dysfunction was primarily pacing-induced ventricular desynchronisation and that the large atrial septal defect might aggravate the condition. However, the antibody-related myocardial injury could not be excluded from the aetiology. Pacing change and atrial septal defect closure were needed to improve the cardiac function. Surgical closure was necessary to treat the atrial septal defect because it was unsuitable for transcatheter closure due to insufficient rims and the small body size of the patient. We thought of avoiding cardiopulmonary bypass use in a situation where antibody-related cardiomyopathy could not be denied and wanted to understand the histological situation of the myocardium. Therefore, we changed the pacing site and mode (from right ventricular outflow to left ventricular apex, VVI to DDD, respectively) and performed endomyocardial biopsy during the thoracotomy of pacing leads reimplantation, where a bioptome was inserted directly into the right atrium through a sheath. The post-operative progress was unremarkable; hence, she was discharged on the 22nd post-operative day. Endomyocardial biopsy revealed no myocyte disarray, vacuolation, or cellular infiltration (Fig 2A–D). Mild interstitial fibrosis and overstretched myofibril were observed, but no massive myocyte loss was detected (Fig 2E, 2F). There was also an increase in the number of mitochondria as an adaptive response. Based on this finding, myocardial damage was assumed to be mild, and recovery of cardiac function after pacing change was expected. Cardiac catheter examination performed 1 year after the alteration of pacing revealed improved left cardiac function (ejection fraction, 67%; left ventricular end-diastolic pressure, 10 mmHg), no pulmonary hypertension, and ratio of pulmonary blood flow to systemic blood flow of 1.8. Based on these data, we concluded that surgical closure was feasible. At 5 years of age, the patient underwent surgical closure of a secundum atrial septal defect. The post-operative course was uneventful, and the patient was discharged on post-operative day 7.

Figure. 2. Microscopic findings. ( a – d ) Light microscopic findings. Myocyte array is maintained with mild interstitial fibrosis. ( a , b ) Elastica van Gieson stain. Arrows pointing collagen deposition. ( c , d ) Masson’s trichrome stain. Arrows pointing collagen deposition. ( e , f ) Electron microscopic findings. Longitudinal section showing overstretched otherwise hypercontractive myofibrils. Increasing and swelling of mitochondria are also shown. ( e ) (Original magnification × 3000) ( f ). (Original magnification × 4000).
Discussion
This case highlighted two clinical issues. First, the cardiac histological findings of a patient with antibody-associated congenital heart block accompanied by a large secundum atrial septal defect and cardiac dysfunction revealed mild myocardial damage. Second, endomyocardial biopsy was useful for decision-making in the repair of an atrial septal defect accompanied by antibody-related congenital heart block and cardiac dysfunction.
We described the histological findings of a patient with antibody-associated congenital heart block accompanied by a large secundum atrial septal defect and cardiac dysfunction for the first time. It is difficult to distinguish the aetiology of cardiac dysfunction after pacemaker implantation associated with antibody-related congenital heart block. Reference Villain, Costedoat-Chalumeau and Marijon2 Descriptions of the histology of antibody-related dilated cardiomyopathy and pacing-induced cardiomyopathy with congenital heart block are limited, and some of the findings were contradicting and non-specific. Reference Udink ten Cate, Breur and Cohen3,Reference Karpawich, Rabah and Haas8 Although it may be difficult to distinguish the aetiology by histological findings, it is important to know the degree of myocardial damage. In our case, there was at most mild myocardial damage, despite severe cardiac dysfunction. In large atrial septal defects, interventricular septal paradoxical motion due to right ventricular volume overload may aggravate ventricular desynchronisation, leading to severe heart failure.
Endomyocardial biopsy was useful for decision-making in treating secundum atrial septal defect accompanied by antibody-associated congenital heart block and cardiac dysfunction. In a previous report of 19 patients with secundum atrial septal defect associated with congenital heart block, three were associated with dilated cardiomyopathy and died. Among the three patients, one with endocardial fibroelastosis died at 2 days, another died at 2.2 years, and the third died after the atrial septal defect operation at 3.4 years. Reference Eronen, Siren and Ekblad4 The cause of post-operative death was unclear, but further deterioration of cardiac function due to cardiac surgery might have been the reason. In another report, a patient with congenital heart block and an atrial septal defect died at age 31 due to dilated cardiomyopathy. She had undergone a surgical septal closure at 21.5 years and pacemaker implantation 1 year after the operation. Reference Eronen9 Although the details are unknown, the surgical septal closure might be associated with the onset of cardiomyopathy. Therefore, it is rational to determine the myocardial condition before intracardiac repair. Based on the histological findings and the course after pacing change, the main cause of heart failure in our patient was presumed to be pacing-induced ventricular desynchronisation. By understanding the histological situation, which was not devastating, we could estimate the perioperative risk and decide on cardiac surgery. If the histological myocardial damage had been catastrophic, we might have avoided surgical septal closure and explored the possibility of percutaneous septal closure when the patient’s body size would have become sufficiently large.
In conclusion, histological findings from the patient with antibody-associated congenital heart block accompanied by a large secundum atrial septal defect and cardiac dysfunction revealed a mild myocardial injury. In our patient, histological findings were useful to speculate the aetiology of heart failure and decide on atrial septal defect treatment.
Acknowledgements
None
Financial support
This research received no specific grant from any funding agency, commercial, or not for profit sectors.
Conflicts of interest
None
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.





