Case report
We report about a 6-weeks-old female infant of body weight 3.4 kg. Following a spontaneous vaginal delivery, the first 3 weeks of the full-term born neonate were uneventful. She was referred from home in a Ross functional class IV with heart rate of 190 beats per minute (bpm), respiratory rate above 70 per minute, oxygen saturation of 91%, blood pressure with small amplitude of 86/60 mmHg associated with a pale skin, and respiratory distress. The clinical symptoms started several days before with sluggishness and feeding difficulties. Further cardiopulmonary examination revealed muffled heart murmurs. Echocardiography was performed immediately that showed a severe depressed left ventricular shortening fraction of 14%, together with bi-ventricular dilated cavities; the right ventricular end-diastolic diameter was 26 mm, and the left ventricular end-diastolic diameter was 14 mm (Fig 1a). The aortic arch seemed to be narrowed, but colour- and pulsed wave-Doppler measurements were without any obstructed blood flow patterns. Aortic coarctation could be excluded; the ductus arteriosus was closed. However, a peri-membranous ventricular septal defect with bi-directional shunting of width 3–4 mm was revealed, together with an 8 mm atrial septal defect secundum type predominating left-to-right shunting. Laboratory data showed elevated Troponin-T (330 pg/ml, cut-off <14 pg/ml) and NT-proBNP (>70,000 pg/ml, cut-off <350 pg/ml for children) values without any serological infection markers, with C-reactive protein of 0.22 mg/dl. Metabolic disorders as a cause for the congestive heart disease, assuming a dilated cardiomyopathy, were excluded. Cytomegalovirus was detected in serum and urine by polymerase chain reaction technique. On the basis of positive viral results, a cytomegalovirus myocarditis was suspected and an oral anti-viral therapy with valganciclovir was initiated, in total, for 8 weeks as treatment. In the paediatric ICU, after a volume depletion was excluded, an anti-congestive therapy was immediately started with continuous infusion of milrinone of 1 µg/kg/minute and levosimendan of 0.1 µg/kg/minute for 24 hours without loading dosage. In addition, intravenous γ-globulinReference Drucker, Colan and Lewis1 was used twice for two consecutive days at a dosage of 1 g/kg/day. Considering a dramatic myocarditis-induced heart failure, β1-receptor blocker, bisoprolol, with a starting dose of 1 × 0.625 mg was additionally initiated to reduce the heart rate and for protecting the cardiac myocytes.Reference Kanda, Adachi and Ohno2–Reference Kindermann, Kindermann and Kandolf4 Bisoprolol therapy was continued combined with the long-acting angiotensin-converting enzyme inhibitor, lisinopril, and the mineralocorticoid-receptor blocker, spironolactone, until the patient fully recovered with a 6-month follow-up.Reference Schranz and Voelkel5 Considering the persistent non-restrictive atrial septum defect, left heart failure related to post-capillary pulmonary hypertension might have been avoided. Cardiac catheterisation performed initially revealed a pre-capillary pulmonary hypertension with systolic and intermittent supra-systemic systolic pressures. The systolic right ventricle pressure was 72 mmHg at rest corresponding to right and left ventricular end-diastolic pressures of 9 mmHg. Considering the pulmonary artery pressures of 72/31 mmHg, the consecutive calculated diastolic pressure gradient was 22 mmHg. Angiographies showed a dilated, low-contracting left ventricle with only less volume-filled aortic vessels, but inconspicuous aortic arch. To relieve the right ventricle pressure workload and to support the severe diseased left ventricular responsible for a sufficient systemic blood flow, the arterial duct was re-opened by guide-wire technique with consecutive balloon dilatation and a coronary stent of 4 × 16 mm (Liberte®, Boston Scientific, Munich, Germany) was placed.Reference Latus, Apitz, Moysich, Kerst, Jux, Bauer and Schranz6 The established right-to-left shunt across the stented duct with a pulsoximetric measured oxygen saturation of about 85–90% in the right arm and 75% in the legs persisted during the initial days. After 10 days the predominant left-to-right shunt became evident, with a slightly improved global heart function. Considering the risk/benefit analysis, myocardial biopsy was not performed because of the right ventricular pressure at systemic level and the objective evidence of cytomegalovirus. After a 14 days follow-up, a second heart catheterisation became necessary. Clinical and left ventricular functional improvement was associated with a reversal of the shunt direction across the stented duct and ventricular septal defect and an increased flow over the atrial septum defect. Therefore, the ductal stent was closed by implantation of a 5 × 4 mm ADO-II-AS and the atrial septal defect by an 8 mm Amplatzer-ASD occluder. Further clinical improvement of the cardiovascular condition was achieved and the infant was discharged home with bisoprolol, lisinopril, and spironolactone as oral anti-congestive medication and an anti-viral drug. After 2 months, the restrictive peri-membranous ventricular septal defect was finally closed using a 6 × 4 mm Amplatzer duct occluder II, when right ventricular pressure values significantly decreased (Fig 2). A further 6 months follow-up revealed normal ventricular dimension and normal functioning of the heart. Clinical condition and echocardiographic data (Fig 1b) correlated with the normalisation of NT-pro-BNP and troponin serum levels (Fig 3).
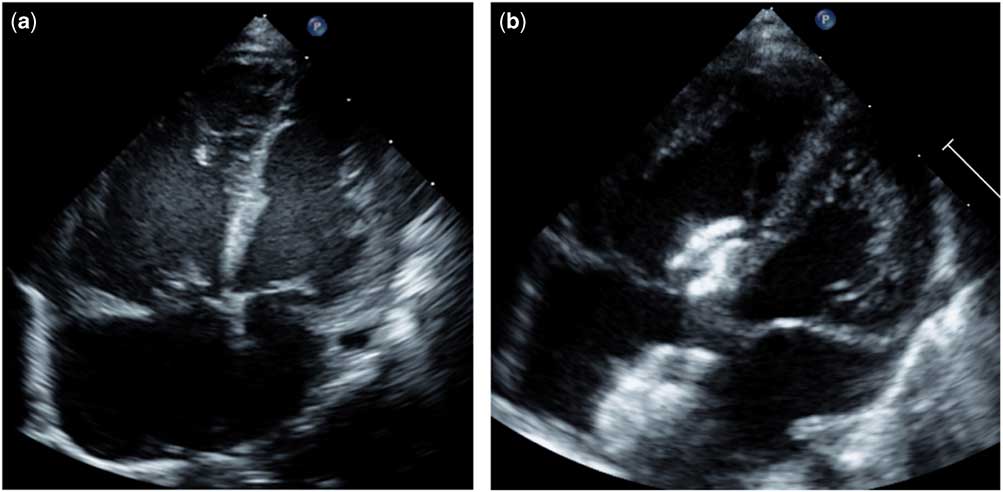
Figure 1 (a) Echocardiographic four-chamber view with bi-ventricular dysfunction and dominant right ventricular dilation (b) Cardiac dimension after recovering.

Figure 2 (a) Lateral fluoroscopy view after occlusion of the atrial septal defect with a 8 mm Amplatzer and 8 mm ASD-occluder; a peri-membranous ventricular septum defect with a 6 mm Amplatzer ADO-II and the stented duct with a 4 mm Amplatzer ADO-II-AS occluder (b) ASD and VSD occluder by echocardiographic imaging. ASD = atrial septal defect; VSD = ventricular septal defect.

Figure 3 Troponin T and NT-pro BNP levels throughout the treatment.
Discussion
In summary, this case report described that severe heart failure in infancy has a high chance of functional regeneration, particularly in context of an inflammatory disease. In bi-ventricular cardiovascular failure associated with pulmonary hypertension, the generation of a fetal-like parallel circulation with an atrial left-to-right and arterial right-to-left shunt performed by transcatheter re-ducting may gain time for specific and anti-congestive treatments in terms of interventional bridging to recover.
On considering pathophysiological-based therapeutic strategies by combining catheter and/or surgical procedures with disease-targeted drug therapies, we might be able to avoid extra-corporal life-support with a higher risk/benefit ratio.Reference Schranz, Akintuerk and Voelkel7
The fact that an infant with a cytomegalovirus myocarditis-associated heart failure and persistent pulmonary hypertension was kept alive with re-ducting was surprising, though not by the natural setting of a non-restrictive atrial septal defect together with a restrictive ventricular septal defect. We could not determine if the persistence of congenital shunts ameliorated or worsened the viral myocarditis. However, unloading the workload of right ventricular pressure by re-ducting, together with improvement of the systemic blood flow on expense of slightly de-oxygenated blood in the lower body, allowed time for recovery.
In conclusion, we were able to successfully treat and protect the heart of a severe diseased infant without extra-corporal life-support by anti-congestive therapy, together with a specific antiviral treatment. From our point of view, the success was only possible by combining additional consecutive interventional procedures which resulted in functional cardiac regeneration, with percutaneous shunt closure of the atrial, ventricular, and arterial level. The hybrid treatment strategy resulted in a rapid normalisation of the patient’s clinical functional class, the myocarditis-associated Troponin-T, and cardiac dysfunctional-related pro-BNP levels, as revealed by echocardiographic measurements.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere.
They confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
The manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. The authors further confirm that the order of authors listed in the manuscript has been approved by all of us and due consideration was given to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. Thus, the authors confirm that the regulations of their institutions concerning intellectual property have been followed.





