Patients with transposition, with concordant atrioventricular and discordant ventriculoarterial connection, used to be surgically palliated with the atrial baffle switch procedure – Senning or Mustard – with redirection of venous return into the heart.Reference Warnes 1 Owing to the fact that dysfunction of the resulting systemic morphologic right ventricle is a common long-term problem, there was a major motivation to attempt anatomic repair with a morphologic left ventricle as systemic pump. In 1975, the first arterial switch procedure with relocation of the coronary arteries was performed by Jatene et al,Reference Jatene, Fontes and Paulista 2 , Reference Jatene, Fontes and Paulista 3 and it has become the current preferred technique, in combination with the Lecompte manoeuvre.
Apparently, these two surgical techniques result in different ventriculoarterial connections with either ventricle acting as the subaortic or subpulmonary pump.
Flow-sensitive four-dimensional velocity-encoded magnetic resonance imaging enables, in addition to blood flow quantification, the visualisation of flow profiles in the heart and great vessels.Reference Markl, Kilner and Ebbers 4 – Reference Valverde, Nordmeyer and Uribe 7 In healthy adults, the systolic flow pattern in the pulmonary trunk is described to be relatively laminar and mostly parallel to the vessel wall,Reference Reiter, Reiter and Kovacs 8 whereas in the aorta an overall right-sided helical pattern is typical.Reference Frydrychowicz, Markl and Hirtler 9 Clinically, these flow profiles are of relevance, as disturbed flow and low or reciprocating shear stress have a negative impact on endothelial cell function and promote atherosclerosis and thrombosis.Reference Chiu and Chien 10 In several scenarios of congenital heart disease, insights into non-physiologic flow patterns were provided.Reference Nordmeyer, Berger, Kuehne and Riesenkampff 5 , Reference Geiger, Markl and Jung 11 , Reference Markl, Geiger and Kilner 12
The aim of this study was to analyse flow profiles in the aorta and the pulmonary trunk in patients with transposition operated by either atrial redirection or arterial switch, and hence differing ventriculoarterial connection and mechanics of the coherent pumping ventricle.
Methods
Patients after surgical correction of transposition without other cardiac malformations who were referred for routine cardiac magnetic resonance between August, 2009 and January, 2012 for measurement of ventricular size and function and/or anatomic and functional assessment of the pulmonary arteries, which were not visible by transthoracic echocardiography, were included in the study. The study was approved by the institutional review board. Written informed consent was given by the patients and/or their legal guardians if patients were under 18 years of age. A total of eight healthy volunteers without cardiac medical history served as controls.
Cardiac magnetic resonance
Data acquisition
Magnetic resonance imaging was performed with a 1.5-T scanner (Philips, Best, The Netherlands) using the sense-cardiac coil. The scan protocol to evaluate ventricular volumes and function by transversal cine images – steady-state free precession sequence, 6-mm slice thickness with zero gap – has been described elsewhere.Reference Beerbaum, Barth and Kropf 13 , Reference Riesenkampff, Schmitt and Schnackenburg 14 As part of the present study, a flow-sensitive four-dimensional velocity-encoded magnetic resonance imaging sequence was performed covering the aorta and the pulmonary trunk. Exemplary scan parameters of this sequence were: field of view 180 × 234 × 80 mm, matrix 128, 32 slices, acquired voxel 2.5 × 2.5 × 2.5 mm, reconstructed voxel 1.7 × 1.7 × 2.5 mm, repetition time 3.6 ms, echo time 2.3 ms, flip angle 5°, retrospective cardiac gating, 25 reconstructed cardiac phases, resulting in a temporal resolution of 34 ms (at a heart rate of 70 bpm), scan time 10 minutes and 45 seconds, velocity encoding 300 cm/second, and number of signal averages 1.
Data analysis
Analysis of cine images included assessment of end-diastolic and end-systolic ventricular volumes and ejection fraction, as well as measurement of the surface area of the ascending aorta and pulmonary trunk, normalised to body surface area, as described elsewhere.Reference Beerbaum, Barth and Kropf 13 , Reference Kutty, Kuehne and Gribben 15 Owing to the oval shape of the pulmonary artery after arterial switch,Reference Massin, Nitsch, Dabritz, Seghaye, Messmer and von Bernuth 16 the vessel cross-sections instead of diameters were measured.
Analysis of the flow-sensitive four-dimensional data sets was performed with the software GTFlow (Release 1.6.8, Gyrotools, Zurich, Switzerland). All data sets were of good quality and suitable for analysis. The systolic flow patterns in the ascending aorta and pulmonary trunk were analysed with regard to the presence and extent of helical flow and vortex formation. The grading included (1) mainly parallel, laminar flow without swirling, (2) overall helicity (spiral flow), (3) regional helices, and (4) vortices with recirculating particles, capturing (4A) <50% or (4B) >50% of the vessel diameter. Helical flow was considered to be the circular motion of blood in the main direction of flow, with overall helicity capturing the whole vessel, and regional helices less than half of the vessel diameter, as described by Frydrychowicz et al.Reference Frydrychowicz, Markl and Hirtler 9
The emitter planes for streamlines to visualise flow in the aorta were placed below the aortic valve, in the aortic sinus, the sinotubular junction, and in the ascending aorta. In the pulmonary arteries, the emitter planes were positioned below and above the pulmonary valve and in the mid-pulmonary trunk.
Statistics
Parameters of ventricular size and function are reported as means and standard deviation, and compared between patients using Student's t-test.
The distribution of the different flow profiles was compared between the two patient groups with different operation techniques and also between each of the two groups and the control group of healthy volunteers using Fisher's exact test. After checking the overall distribution of all flow profiles, separate analyses for each special profile were conducted, in order to detect the profiles responsible for the group differences.
The analyses have been carried out with SPSS, version 19. In all tests, p-values of <0.05 were considered as significant.
Results
A total of 29 patients were assessed: n = 17 patients after atrial redirection – 14 after Senning and three after the Mustard procedure – and n = 12 patients after the arterial switch operation. Surgery had been performed at different centres. All patients were in sinus rhythm at rest, and further characteristics are listed in Table 1. Owing to the era difference between the two operative techniques, patients after atrial redirection were older at the time of cardiac magnetic resonance imaging than patients after the arterial switch procedure.
Table 1 Characteristics of patients and healthy controls.

bpm = beats per minute; CMR = cardiovascular magnetic resonance; na = not applicable
Values are presented as mean and standard deviation
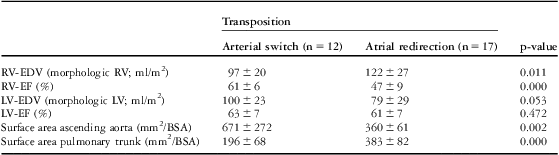
Results of ventricular size and function and vessel dimensions are listed in Table 2. Right ventricular end-diastolic volumes were enlarged and ejection fraction reduced after atrial redirection, and after the arterial switch procedure left ventricular volumes were slightly enlarged. These results are in line with other investigators.Reference Grotenhuis, Ottenkamp and Fontein 17 , Reference Grothoff, Fleischer and Abdul-Khaliq 18 In patients after the arterial switch procedure, the ascending aorta was larger and the pulmonary trunk was smaller compared with patients after atrial redirection.
Table 2 Parameters of ventricular size and function and vessel size.
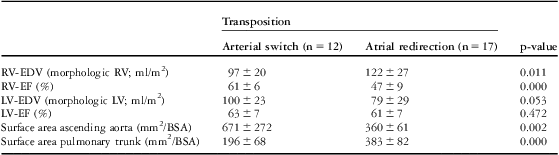
BSA = body surface area; LV = left ventricle; LV-EDV = left ventricular end-diastolic volume; LV-EF = left ventricular ejection fraction; n = number of patients; RV = right ventricle; RV-EDV = right ventricular end-diastolic volume; RV-EF = right ventricular ejection fraction
Values are presented as mean and standard deviation
Flow analysis by streamlines
Aorta
Healthy controls
In the ascending aorta, overall helicity was present in all healthy volunteers, without any vortex formation (Fig 1).

Figure 1 Craniolateral view of the streamlines of the aorta (left) and pulmonary arteries (right), colour-coded according to velocity, in a healthy control. The streamlines in the aorta show a spiral pattern, whereas in the pulmonary arteries they are mainly parallel.
Arterial switch patients
The normal helical pattern, which is the overall helicity, was missing in the ascending aorta of all patients after the arterial switch procedure. Regional helices or vortices were present in 83% of patients (Fig 2). The details are presented in Table 3.

Figure 2 Lateral view of the streamlines of the aorta (right) and pulmonary arteries (left), colour-coded according to velocity, in a patient with transposition after the arterial switch procedure with the Lecompte manoeuvre. The streamlines in the pulmonary arteries are mainly parallel – visible are the pulmonary trunk and the left pulmonary artery. In the dorsal ascending aorta, a vortex type 4A, capturing <50% of the vessel diameter, is evident (arrowheads), whereas the remaining aortic streamlines are mainly parallel.
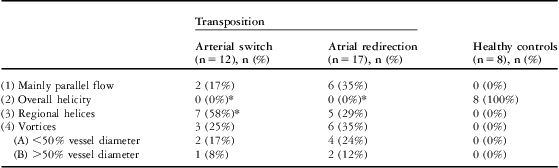
Table 3 Results of streamline flow analysis relating to parallel and helical flow and vortex formation in the ascending aorta.

n = number of patients
Presented are the absolute numbers of patients with the respective flow profiles, and in parentheses the calculated percentage of all patients of the group
*Significant difference compared with the control group
Atrial redirection patients
There was absence of the normal overall helicity in the ascending aorta of all patients after atrial redirection. In 64% of patients, regional helices or vortices were present, mostly dorsally in the ascending aorta (Fig 3). Table 3 summarises the findings.

Figure 3 Lateral view of the streamlines of the aorta (left) and pulmonary arteries (right), colour-coded according to velocity, in a patient with transposition after atrial redirection. The streamlines in the aorta are mainly parallel, whereas in the pulmonary trunk a vortex type 4B, capturing >50% of the vessel diameter, is visible (arrowheads).
In the statistical comparison with Fisher's exact test, there were no significant differences of flow profiles between the two patient groups, although regional helices were present in a greater number of the arterial switch patients (58% versus 29%). However, both patient groups differed highly significantly from the healthy controls. In these latter comparisons, the differences were particularly present for flow profile (2), the overall helicity, and partly for profile (3), the regional helices.
Pulmonary trunk
Healthy controls
Flow was mainly parallel in the pulmonary trunk of all healthy volunteers (Fig 1).
Arterial switch patients
Flow was mainly parallel in 83% of patients. Minor vortices were present in 17% of patients (Fig 2). Table 4 summarises the findings.
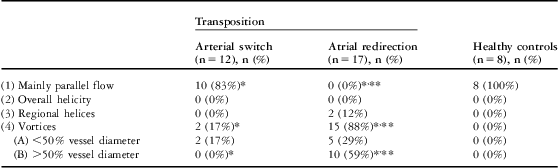
Table 4 Results of streamline flow analysis relating to parallel and helical flow and vortex formation in the pulmonary trunk.
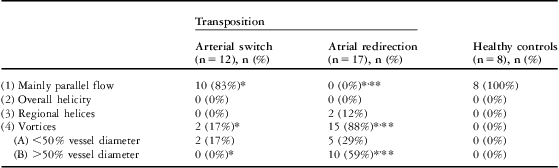
n = number of patients
Presented are the absolute numbers of patients with the respective flow profiles, and in parentheses the calculated percentage of all patients of the group
*Significant difference between the two patient groups
**Significant difference compared with the control group
Atrial redirection patients
In the pulmonary trunk, vortex formation was visible in 88% of patients after atrial redirection (Fig 3). Details and additional findings are presented in Table 4.
The statistical tests show highly significant differences for the atrial redirection group compared with patients after atrial redirection and healthy controls, based on differences in the occurrence of flow profile (1), the mainly parallel flow, and flow profile (4), particularly (4B) the vortices. Arterial switch patients and healthy controls showed no significant differences.
Discussion
This study reveals major differences in the systolic blood flow patterns in the ascending aorta and pulmonary trunk in patients with operated transposition compared with healthy controls and depending on the surgical technique, atrial redirection or arterial switch.
The overall helicity, which is present in the aorta of healthy individuals, is missing in all patients independent of the performed surgery. In the pulmonary trunk, where a mostly parallel flow pattern is physiologic, extensive vortices are present in patients after atrial redirection.
These observed differences have several possible reasons.
Vortices in pulmonary arteries
Similar vortices as seen in our study in the pulmonary trunk after atrial redirection have been observed in the pulmonary trunk of patients with pulmonary hypertension.Reference Reiter, Reiter and Kovacs 8 However, whether these vortices are a result of the elevated pressure in the pulmonary arteries itself or caused by altered mechanics of the pressure-loaded right ventricle is unknown, and warrants further investigation. In patients with transposition after atrial redirection, the response of the morphologic right ventricle to high afterload, acting as subaortic ventricle, is a shift from longitudinal to circumferential shortening,Reference Pettersen, Helle-Valle and Edvardsen 19 which could have an impact on vortex formation.
The elevation of pulmonary vascular resistance and of pulmonary artery pressure in several patients with transposition is a known problem.Reference Wilson, Clarkson and Barratt-Boyes 20 , Reference Yehya, Lyle, Pernetz, McConnell, Kogon and Book 21 In three of our patients, pressure data from invasive catheterisation were available, and were at normal values in two and slightly elevated in one patient. Higher pressure did not provoke greater vortices, as a vortex type 4A – capturing <50% of the vessel diameter – was present in the pulmonary trunk of the patient with elevated pulmonary artery pressure, and a type 4B vortex – capturing >50% of the vessel diameter – in the patients with normal pressures. Analysis of a greater number of patients on the basis of pressure values, ventricular mechanics, and flow patterns to clarify these relationships is desirable.
Aortic properties
Characteristics of the vessels themselves such as geometry and wall conditions impact flow.
Aortic spiral pattern
The normal heart has a clockwise spiral pattern of the outflow tracts and of the great arteries.Reference Marino and Corno 22 The helical or spiral flow pattern, present in the human aorta in healthy subjects, results, at least partly, from the right-handed twist of the great arteries, the curvature of the arch, and the pulsatility of flow, which was demonstrated by Kilner et alReference Kilner, Yang, Mohiaddin, Firmin and Longmore 23 by flow simulations in flat and twisted arches. In patients with transposition, this spiral pattern is missing as part of the disease,Reference Marino and Corno 22 and hence the missing overall helicity in all of our studied patients could perhaps be explained by the missing looping. An alternative surgical arterial switch technique with restoring the spiral relationship of the great arteries, as proposed and presented by Chiu and colleagues,Reference Chiu, Huang and Chen 24 , Reference Li, Leow and Chiu 25 might be useful to restore the overall helicity. This spiral flow has beneficial effects on cardiac work and on protecting the arterial wall from atherogenesis, as demonstrated in aortic segments of rabbits.Reference Ding, Fan, Deng, Zhan and Kang 26 In this study, swirling flow resulted in reduced uptake of atherogenic low-density lipoproteins by the arterial wall.
The influence of aortic arch geometry, diameter, and age on the flow profile of the aorta has been studied recently, and geometric factors showed a lesser impact on blood flow patterns than age and diameter.Reference Frydrychowicz, Berger and Munoz Del Rio 27 According to this study, the differing geometry of the aortic arch depending on the surgical technique, with a more acute angle or gothic arch in the arterial switch patients,Reference Agnoletti, Ou and Celermajer 28 would not impact flow patterns significantly. Nevertheless, in our atrial redirection patients, who had greater diameters of the ascending aorta and were older, helices were found to a lesser extent, and helicity is reported to be less common with age.Reference Frydrychowicz, Berger and Munoz Del Rio 27 Further analysis is needed with age- and gender-matched controls and equal vessel diameters.
Aortic vessel wall
There are known vessel wall abnormalities in patients with transposition, which are assumed to have their origin in the altered amount of oxygen in the great arteries in the prenatal period.Reference Rudolph 29 Reduced elasticity of the proximal aorta has been observed in patients after the arterial switch operation,Reference Grotenhuis, Ottenkamp and Fontein 17 and increased carotid artery stiffness in all operated patients by either surgical method, the atrial redirection, or arterial switch procedure.Reference Mersich, Studinger, Lenard, Kadar and Kollai 30 Whether missing spiral flow impacts these vessel wall abnormalities or vice versa needs to be evaluated. As mentioned above, disturbed flow and low or reciprocating shear stress have a negative impact, as they promote atherogenesis and thrombogenesis,Reference Chiu and Chien 10 and hence an undisturbed flow is desirable for all patients to optimise endothelial cell function. Arterial aneurysm formation is also associated with low shear stress and disturbed flow.Reference Chiu and Chien 10 The manner in which the enlarged aortic diameters in patients with transposition after arterial switch are related to the present vortices (Fig 2) needs to be assessed in future studies.
Ventricular mechanics
The flow patterns could potentially be related to the mechanics of the coherent ventricles. In the normal left ventricle, torsional biomechanics are prominent, whereas in the normal right ventricle longitudinal contraction is greater.Reference Carlsson, Ugander, Heiberg and Arheden 31 In healthy individuals, the torsional contraction pattern of the left ventricle may play a role in the creation of the overall helicity in the aorta, and the longitudinal contraction mode of the right ventricle might induce the mainly parallel flow in the pulmonary trunk. In patients with transposition after atrial redirection, the observed differing flow patterns could be explained by the anatomy with still discordant ventriculoarterial connection. These hypotheses warrant further study.
In summary, flow profiles in the aorta and pulmonary trunk of patients with transposition operated by the two surgical techniques differ from normal and among themselves. In addition to clarifying causes and effects, the significance of the findings and influencing factors need to be elucidated in combination with clinical consequences in future studies.
Limitations
In this study, owing to the historical difference in surgical techniques, different age groups were investigated, and age- and gender-matched controls are lacking.
Conclusion
Blood flow patterns differ substantially between the groups, without overall helicity of aortic flow in all patients, and pronounced vortex formation in the pulmonary trunk in patients after atrial redirection. In addition to differing mechanics of the coherent pumping ventricles, the missing looping of the aorta in transposition might be a reason.
Acknowledgement
This work was supported by the German Federal Ministry of Education and Research (BMBF), grant 01EV0704. The authors thank Alireza Khasheei for technical assistance. There are no disclosures to be made for any conflicts of interest.