Fibromuscular dysplasia is a rare non-inflammatory, non-atherosclerotic disease of small- to medium-sized arterial vessels. Fibromuscular dysplasia mostly occurs at the renal arteries,Reference Slovut and Olin 1 although other anatomical sites such as carotid, cerebral, brachial, or coronary arteries have also been reported.Reference Olin and Sealove 2
Case report
In this study, we report the case of a 1-day-old mature boy born by caesarean section at the 38th week of gestation after presenting with pathological cardiotocography and foetal tachycardia. Postnatally, the infant developed severe left ventricular dysfunction with immediate need for catecholamines. As his mother showed elevation of infection parameters, an antibiotic therapy was additionally initiated. Echocardiography of the infant revealed a hypokinetic to akinetic left ventricular wall, whereas the right ventricle appeared to be functionally normal. The complete left coronary artery was not traceable at coronary angiography (Fig 1), although aortic outbound of the left coronary artery could be confirmed in the digital subtraction angiography. Despite extensive treatment including extracorporeal membrane oxygenation and high-dose catecholamine application, the infant died 3 days after birth due to cardiac insufficiency. A right ventricular myocardial biopsy taken at day 1 after birth did not reveal significant histological signs of inflammation or congenital structural defects of the cardiomyocytes. Autopsy, however, revealed a distinct, ~2–3-day-old myocardial infarction affecting large parts of the left ventricle and septum (Fig 2a). The right coronary artery did not display any pathological differences, neither macroscopically nor histologically (Fig 2b). Despite a negative cardioangiograpy – where the left coronary artery was not demonstrable – the complete left coronary artery including all the major branches was present at autopsy; however, the left anterior descending exhibited a sharply demarcated narrowing in the middle to distal part (Fig 2c). Histologically, only slight defects in the endothelial lining were detectable, showing focal homogeneous fibrin deposits adherent to the endothelium of the distal left anterior descending, possibly resembling residual parts of a larger thrombus (Fig 2d). Remarkably, a concentric extensive accumulation of collagen in the media and adventitial layer as cause of luminal narrowing was notable (Fig 2e and f). The architecture of the vessel wall, however, was accurate (Fig 2g). No signs of vascular or myocardial inflammation were observed. Additional histochemical staining (Fig 2h) did not reveal pathological deposits within the media. Therefore, an isolated fibromuscular dysplasia of the left anterior descending – peri-medial subtype – was diagnosed, resulting in an extensive myocardial infarction in the supply territory of the left anterior descending with subsequent heart failure as cause of death. Other anatomical parts of the heart including valves, atria, and epicardium, as well as both the lungs and other larger vessels – for example, aorta – appeared to be normal.

Figure 1 Digital subtraction angiography of the coronary arteries. The right coronary artery as well as the aortic outbound of the left coronary artery are both clearly detectable; however, the complete left coronary artery distal of the outbound was not traceable.
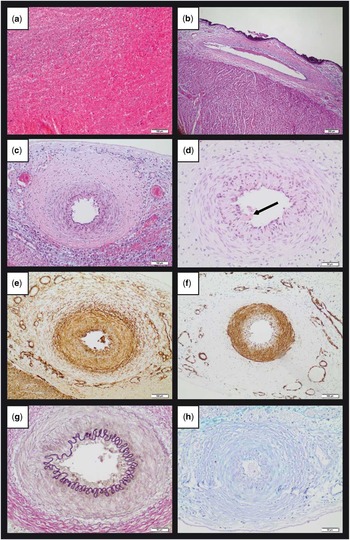
Figure 2 ( a ) Approximately 2–3-days-old left ventricular myocardial infarction with haemorrhage and incipient inflammatory demarcation (HE; scale bar: 100 µm). ( b ) Normal histological appearance of the right coronary artery (HE; scale bar: 200 µm). ( c ) Concentric fibrous narrowing of the left anterior descending (HE; scale bar: 100 µm). ( d ) Slight endothelial defects including adherent residual parts of thrombotic material (arrow) within the left anterior descending (HE; scale bar: 50 µm). ( e ) Immunohistochemical staining for collagen I, highlighting fibrous tissue surrounding the medial layer of the left anterior descending (scale bar: 100 µm). ( f ) Immunohistochemical staining for α smooth muscle actin highlighting the medial layer of left anterior descending (scale bar: 100 µm). ( g ) Elastica van Gieson histochemical staining showing regular distribution of elastic fibres within the left anterior descending (scale bar: 50 µm). ( h ) Histochemical staining confirms the absence of detectable pathological deposits (AB-PAS; scale bar: 50 µm). AB-PAS=alcian blue and periodic acid Schiff; HE=haematoxylin and eosin.
Discussion
Most patients affected by coronary fibromuscular dysplasia present at mid-age and are predominantly women.Reference Olin, Froehlich and Gu 3 A genetic predisposition for fibromuscular dysplasia is being discussed at present, as ~10% of first-degree relatives are usually affected. Fibromuscular dysplasia can be asymptomatic over years and sometimes challenging for clinicians to diagnose. Especially in the coronary arteries of patients where typical signs of fibromuscular dysplasia are not always present, initial diagnosis and subsequent accurate treatment might be postponed for years. Therefore, a significant number of fibromuscular dysplasia is primarily detected during autopsy in cases with history of sudden death.
Although clinically manifest fibromuscular dysplasia with coronary artery involvement is believed to be rare, its true prevalence is still unknown, due to a lack of specific coronary findings or symptoms before an acute event.Reference Michelis, Olin, Kadian-Dodov, D’Escamard and Kovacic 4 Fibromuscular dysplasia can occur at any age. To date, only few reports describe fibromuscular dysplasia in the coronary arteries as the cause of sudden death in infants and young children,Reference Imamura, Yokoyama and Kikuchi 5 – Reference Tullu, Vaideeswar, Bhandare and Deshmukh 7 mostly affecting children in their first 2 years of life, with the age of onset ranging from 3 to 16 months. Clinical complications of fibromuscular dysplasia, as seen in the presented case, typically include myocardial infarction and severe ventricular dysfunction caused by angiospasm or thrombotic plug of the affected vessel section.Reference Camuglia, Manins, Taylor and Hengel 8 , Reference Ogawa, Nomura and Komatsu 9 Interestingly, a slight predominance of the larger coronary vessels affected by fibromuscular dysplasia, and hereby especially of the left anterior descending, has been observed,Reference Huizar, Awasthi and Kozman 10 whereas no difference between affection of the epi- or intra-myocardial parts could be seen.
With special regard to this case presented here, maybe the clinical diagnosis of fibromuscular dysplasia could have possibly been established at day 1 after birth when the right ventricular heart biopsy was taken to rule out myocarditis and as the left coronary arteries intraoperatively appeared to be narrowed; however, according to histological changes seen in the myocardium, the onset of myocardial infarction must have been much earlier, and thus most probably clinical outcome would have been the same even if fibromuscular dysplasia was detected earlier.
In conclusion, fibromuscular dysplasia of the coronary arteries is a rare but probably underestimated cause of death. Clinicians should be aware that fibromuscular dysplasia can occur at any age, and in most cases diagnosis might be challenging. Nevertheless, fibromuscular dysplasia should be routinely considered after inflammatory and thrombotic causes, as well as after congential heart failures have been ruled out. Especially abnormal coronary angiography findings such as angiographic lack of one coronary artery might be the only clue that leads to the correct diagnosis. Early clinical diagnosis can be benefitial for the patients as treatment options exist. Nevertheless, patients’ outcome presenting with signs of advanced myocardial damage (myocardial infarction/severe ventricular dysfunction) is mostly poor and will not significantly differ from other causes. If fibromuscular dysplasia appears in children, mostly young children and infants below the age of 2 are affected; therefore, neonatologists, especially, should be aware of fibromuscular dysplasia of the coronary arteries.
Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethical Standards
This study was performed according to the declaration of Helsinki. Written informed consent was obtained from the parents.





