Critically ill children with cardiac disease comprise a distinct sub-group of infants whose course is frequently characterised by longer mechanical ventilation, more complicated intensive care unit stays, and complex cardiopulmonary interactions.Reference Cox, Piedmonte and Drummond-Webb 1 , Reference Kanter, Bove and Tobin 2 However, post-operative management of children undergoing congenital heart surgery has improved over the years. Mechanical ventilation is discontinued and patients are extubated when the underlying reasons for intubation and mechanical ventilation are resolved. Recognition of children who can be successfully extubated after cardiac surgery is a complex decision-making process. Extubation failure after prolonged mechanical ventilation in children is quite common and is seen in 22–28% of premature babies, 15–20% of critically ill children, and 10% of children after cardiac surgery.Reference Harrison, Cox, Davis, Piedmonte, Drummond-Webb and Mee 3 – Reference Johnston, de Carvalho, Piva, Garcia and Fonseca 8
With advances in anaesthesia management, cardiopulmonary bypass, and surgical techniques, there has been a general trend towards early extubation in children after cardiac surgery. The risks of early and untimely extubation are manifold.Reference Laussen and Roth 9 – Reference Vricells, Dearani and Grundy 11 Extubation failure requiring emergent reintubation may cause significant haemodynamic instability, unnecessary airway trauma, increased risk for nosocomial infections, and prolonged duration of mechanical ventilation and intensive care unit length of stay.Reference Cox, Piedmonte and Drummond-Webb 1 – Reference Khan, Brown and Venkataraman 4 Existing literature on extubation failure is currently limited to heterogeneous patient populations undergoing uncomplicated repairs.Reference Laussen and Roth 9 – Reference Schuller, Bovill, Nijveld, Patrick and Marcelletti 13 This study was undertaken to investigate incidence, aetiology, and outcomes of extubation failure in infants with modified Blalock–Taussig shunt-dependent pulmonary blood flow. A second objective of this study was to determine the haemodynamic effects of transition of positive pressure ventilation to spontaneous breathing in infants with extubation failure.
Methods
Setting and patients
We performed a single-centre retrospective observational study in a 15-bed paediatric cardiovascular intensive care unit at a tertiary academic children's hospital during the period January, 2005 to March, 2011. The Institutional Review Board of the University of Arkansas Medical Sciences approved the study, and the need for informed consent was waived. The study included all infants ⩽6 months of age who underwent modified Blalock–Taussig shunt at the Arkansas Children's Hospital. Patients excluded from the study were infants undergoing Norwood operation or Damus–Kaye–Stansel with modified Blalock–Taussig shunt, infants operated upon in outside institutions and subsequently transferred to our institution, infants with unplanned extubation, infants with tracheostomy, infants extubated directly onto non-invasive ventilation, and infants with the “Do Not Resuscitate” order. We identified the potential patients by querying the departmental surgical database and the hospital medical records.
Airway management
All patients in our cohort were intubated with either 3.5-mm or 4.0-mm cuffed endotracheal tube (Mallinckrodt Incorporation, Hazelwood, Missouri, United States of America) and ventilated with a Servo-I® ventilator (Maquet Incorporation, Wayne, New Jersey, United States of America). The initial mode of ventilation was pressure-regulated volume control. Once the patient was breathing spontaneously and ready for weaning, the ventilator mode was switched to volume support for at least 30 minutes before extubation. Tracheal extubation was performed following a standardised protocol for ventilator weaning in our cardiovascular intensive care unit: fraction of inspired oxygen in a gas mixture ⩽0.4 to maintain systemic oxygen saturation ⩾75–87% as measured by pulse oximetry, peak inspiratory pressure of ⩽20 cm H2O, and/or positive end-expiratory pressure of ⩽6 cm H2O. All patients included in the study were extubated to humidified high flow nasal cannula (Fischer & Paykel Incorporation, Auckland, New Zealand), with peak flow pressures varying between 6 and 10 L/minute. All patients were continuously monitored by a member of the cardiovascular intensive care unit medical team before extubation for respiratory rate, heart rate, transdermal oxygen saturation, the adequacy of chest wall excursion, and use of accessory respiratory muscles. According to the routine clinical practice in our cardiovascular intensive care unit, patients were completely weaned off the sedation and analgesic infusions before extubation if the duration of mechanical ventilation was <5 days. If the duration of mechanical ventilation was ⩾5 days, the patient remained on these infusions in the peri-extubation period; however, the dosages were reduced to half or less than half depending on the patient's wakefulness and team's comfort levels.
Patients who failed extubation were reintubated if they met one or a combination of the following criteria: (1) presence of clinical signs of respiratory fatigue and severe respiratory distress, (2) worsening hypercarbia (increase of ⩾20% from the baseline value) and/or hypoxaemia (decrease of ⩾20% from the baseline value), (3) inability to clear airway/oral secretions, (4) haemodynamic decompensation, (5) cardiorespiratory arrest, (6) Glasgow coma scale of <8, or (7) inability to maintain adequate airway patency owing to neurological impairment. The decision to extubate or reintubate each patient was made by the cardiovascular intensive care unit attending and team caring for the patient.
Technique for non-invasive ventilation
The two modalities of non-invasive ventilation we utilised were continuous positive airway pressure and bi-level positive airway pressure. Positive end-expiratory pressure was initiated with a back-up rate in all of our patients at 4–5-cm H2O and increased as necessary, up to a maximum of 10–12-cm H2O if no improvement in oxygen saturation or arterial oxygen content was achieved. Inspiratory positive airway pressure was initiated at 6–8-cm H2O and gradually increased to a maximum of 18–20-cm H2O if no improvement in clinical status or ventilation was achieved. We provided non-invasive ventilation to patients using nasal prongs, a nasal mask, or a facial mask fitted appropriately according to the patient's age and size to achieve maximum comfort and minimum air leak. We placed a nasogastric tube in all patients before initiation of non-invasive ventilation in order to prevent gastric distension and emesis. A protective patch was placed over the skin of the nasal bridge (Duoderm®, Bristol Myers-Squibb, New York, New York, United States of America) in patients with tight-fitting facial masks to avoid skin breakdown. The head of the bed was elevated to 45° in all patients to reduce the risk of aspiration.
Study definitions and data collection
For study purposes, failed extubation was defined as the need for positive pressure ventilation within 96 hours of an extubation attempt. The need for positive pressure ventilation could be in the form of invasive ventilation needing reintubation or non-invasive ventilation such as continuous positive airway pressure or bi-level positive airway pressure. Of note, infants extubated directly to non-invasive ventilation were excluded from the study. For each patient, the following variables were collected: age, gender, weight, prematurity, associated diagnoses – chronic lung disease, dysrhythmia, pulmonary hypertension, chromosomal abnormality, underlying neuromuscular disease – airway diagnoses – tracheobronchomalacia, tracheal stenosis – and status of intubation before surgery. The operating room variables collected included: cardiopulmonary bypass time, cross-clamp time, inotrope score,Reference Ko, Lin, Chen, Wang, Lin and Chen 14 lactate, and mechanical support in the form of extracorporeal membrane oxygenation at the time of leaving the operating room. The data collected on variables immediately before extubation included administration of intravenous glucocorticoids within 24 hours of extubation, need for dialysis, presence of chylothorax and/or pleural effusion, diaphragm paralysis, sepsis, necrotising enterocolitis, and ventricular ejection fraction. The laboratory indices collected before extubation included arterial blood gas, white blood cell count, haematocrit, blood urea nitrogen, serum creatinine, and lactate. We also collected data on the number of sedation and analgesic infusions at the time of extubation and number of sedation and analgesic rescue boluses given to the patient 24 hours before extubation. For study purposes, sedation and analgesic infusions for our study included midazolam, morphine, fentanyl, and dexmedetomidine infusions. Sedation rescue boluses included midazolam, lorazepam, ketamine, chloral hydrate, and diphenhydramine, whereas analgesic rescue boluses included fentanyl and morphine. We also collected data on days of mechanical ventilation and days of inotropic support before extubation, hours on volume support mode and time to extubation after cardiac surgery.
The cardiorespiratory variables collected before extubation and immediately after extubation included heart rate, respiratory rate, mean arterial blood pressure, central venous pressures, and oxygen saturations. Tissue oxygenation was assessed using near infrared spectroscopy using oxygen sensors on the forehead and abdomen. Data for all these variables were collected for 6 hours before extubation and 6 hours after the extubation, and hourly mean was represented as either “pre-extubation” or “post-extubation” value. Owing to limited data availability, pulmonary hypertension was considered as a categorical variable and assessed on the basis of clinical impression, echocardiogram, or cardiac catheterisation. Pulmonary hypertension was defined as pulmonary artery pressure greater than or equal to systemic arterial pressure, as estimated through echocardiography or cardiac catheterisation. The criteria for organ dysfunction were based on International Pediatric Sepsis Consensus Conference guidelines.Reference Goldstein, Giroir and Randolph 15 “Lung disease” as the cause of extubation failure included presence of atelectasis or consolidation, with or without the presence of chylous or pleural effusion. As a routine practice in our unit, patients receiving positive pressure ventilation receive chest radiograph every morning. For study purposes, the official radiologist interpretation was used to differentiate consolidation from atelectasis on chest radiographs. Any patient with suspicion of diaphragmatic paresis or paralysis on routine chest radiograph received chest ultrasound to evaluate for diaphragmatic mobility. “Cardiac dysfunction” as the cause of extubation failure included low ventricular ejection fraction with or without echocardiographic evidence valvular regurgitation. Qualitatively good ventricular systolic function or an ejection fraction ⩾55% as determined by an echocardiographer blinded to the two groups constituted a good ejection fraction for study purposes. Clinical outcome evaluated for all patients included the success or failure of extubation, cardiovascular intensive care unit length of stay, hospital length of stay, and mortality.
Statistical analysis
Continuous variables are presented as the median (Q1, Q3), where Q1 is the 25th percentile and Q3 is the 75th percentile, whereas categorical variables are presented as numbers and percentages. p-values were calculated using Pearson's chi-square test or Fisher's exact test of independence for categorical variables and Wilcoxon rank-sum test for continuous or ordinal variables. A p-value of ⩽0.05 was considered significant. Comparisons of haemodynamic variables for pre-extubation and post-extubation values were performed within the extubation failure and extubation success groups using the Wilcoxon matched-pairs signed-rank test. A Bonferroni correction was applied to correct for the two-subgroup comparisons per variable. Analyses were performed using STATA/MP, version 11.1 software (Stata® Corp LP, College Station, Texas, United States of America), and Harrell's RMS package in R (available from biostat.mc.vanderbilt.edu/rms).
Results
During the study period, 55 patients qualified for inclusion in the study. The median age of patients at extubation was 19 days (12 days, 22 days) and median weight of patients was 3.6 kg (3.02 kg, 4.26 kg). In all, 38% (21/55) of the patients were intubated before surgery. Extubation failure occurred in 27% (15/55) of all patients. Median duration of mechanical ventilation was 4 days (1 day, 10.5 days), median cardiovascular intensive care unit length of stay was 11 days (6.5 days, 23.5 days), and the median hospital length of stay was 30 days (14 days, 48 days). Overall in-hospital mortality was 7% (4/55).
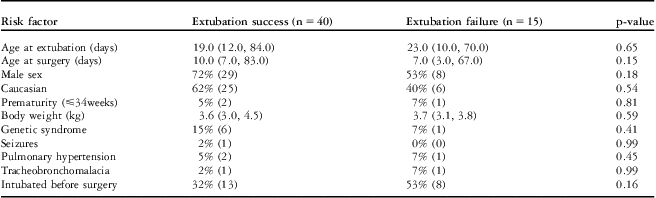
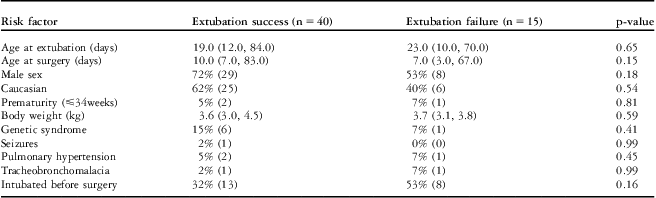
The following patients were included in our study: 32% (18/55) with tetralogy of Fallot and pulmonary atresia; 14.5% (8/55) with pulmonary atresia and intact ventricular septum; 12.5% (7/55) with double-outlet right ventricle and mitral atresia; 12.5% (7/55) with malaligned complete atrioventricular canal and hypoplastic – right or left – ventricle; 11% (6/55) with tricuspid valve atresia, with normal or transposed great arteries; 9% (5/55) with Ebstein's anomaly; and 7% (4/55) with double-inlet right ventricle, with normally related or transposed great arteries. The risk factors and timing of extubation failure is shown in Table 1. Of the 15 patients with extubation failure, 10 patients needed reintubation; seven patients received non-invasive ventilation, of which two were reintubated; and five patients received continuous positive pressure ventilation without getting reintubated. There were three patients who had extubation failure in the first 2 hours after extubation, nine patients in the 2–24-hour period, and three patients in the 24–96-hour period. There were eight patients who were extubated in the second attempt after the first extubation failure, with a median duration of mechanical ventilation of 2 days (1 day, 6 days). There was one patient with extubation failure who was never extubated and died within the hospital before hospital discharge and another patient who required diaphragmatic plication before successful extubation.
Table 1 Risk factors and timing of extubation failure in the study patients.

Table 2 demonstrates demographics and pre-existing conditions for patients in our study. The patients in both groups were similar in age, sex, and body weight at the time of surgery. All pre-existing conditions were also similar in the two groups. Table 3 demonstrates intra-operative variables and post-operative complications for patients in the study. There were no differences in intra-operative variables and post-operative complications among the two groups. Table 4 demonstrates variables in the peri-extubation period, as well as outcomes of the patients in the two groups. Pulmonary oedema, reduced systemic ventricular function, increased number of sedation drips, and increased number of sedation and analgesic boluses in 24 hours before extubation were associated with extubation failure.
Table 2 Baseline demographics and pre-existing conditions for patients undergoing the Blalock–Taussig shunt procedure.
Continuous variables are summarised by the triplet of quartiles 50th (25th and 75th)
Categorical variables are summarised as percent (n)
p-values are based on the Wilcoxon–Kruskal–Wallis test for continuous and ordinal data, and Pearson or Fisher's exact test for categorical variables
Table 3 Intra-operative variables and post-operative complications for the patients in the study

BT = Blalock–Taussig; CPB = cardiopulmonary bypass time; ECMO = extracorporeal membrane oxygenation; OR = operating room
Continuous variables are summarised by the triplet of quartiles 50th (25th and 75th). Categorical variables are summarised as percent (n)
p-values are based on the Wilcoxon–Kruskal–Wallis test for continuous and ordinal data, and Pearson or Fisher's exact test for categorical variables
*Inotrope score: dosages of dopamine + dobutamine (in μg/kg/minute) + [dosages of epinephrine + norepinephrine + isoproterenol (in μg/kg/minute)] × 100 + dosages of milrinone (in μg/kg/minute) × 15
Table 4 Peri-extubation and outcome variables for the study patients.

CICU = Cardiac Intensive Care Unit
Continuous variables are summarised by the triplet of quartiles 50th (25th and 75th)
Categorical variables are summarised as percent (n)
p-values are based on the Wilcoxon–Kruskal–Wallis test for continuous and ordinal data, and Pearson or Fisher's exact test for categorical variables
*Refer to number of boluses 24 hours before extubation.
Table 5 demonstrates changes in cardiopulmonary parameters and tissue oxygenation using near infrared spectroscopy following conversion of positive pressure ventilation to spontaneous breathing. We compared these variables among extubation failure and extubation success groups both before and after extubation. Among the patients with extubation failure, heart rate, respiratory rate, and mean arterial blood pressure were elevated after removal of positive pressure ventilation. These patients also needed higher fraction of inspired oxygen after extubation. Among the patients with extubation success, there was an increase in heart rate, respiratory rate, and mean arterial blood pressure after removal of positive pressure ventilation. Inotrope score decreased in this group after extubation. Despite no need for higher fraction of inspired oxygen after extubation, there was a significant increase in partial pressure of oxygen in arterial blood. We also compared the changes in cardiopulmonary variables in the peri-extubation period between extubation failure and extubation success groups. We found that children with extubation failure needed a higher fraction of inspired oxygen after removal from positive pressure when compared with the extubation success group. Increase in heart rate and respiratory rate after removal from positive pressure was significantly higher in infants with extubation failure. However, increase in mean arterial blood pressure was higher in patients with extubation success.
Table 5 Changes in cardiopulmonary parameters and tissue oxygenation following conversion of positive pressure ventilation to spontaneous breathing

CVP = central venous pressure; FiO2 = fraction of inspired oxygen in a gas mixture; NIRS = near infrared spectroscopy; PaCO2 = partial pressure of carbon dioxide in arterial blood; PaO2 = partial pressure of oxygen in arterial blood
Continuous variables are summarised by the triplet of quartiles 50th (25th and 75th)
Categorical variables are summarised as percent (n)
p-values are based on the Wilcoxon–Kruskal–Wallis test for continuous and ordinal data, and Pearson or Fisher's exact test for categorical variables
On comparing infants undergoing extubation failure with extubation success, there was no difference in the duration of inotropic support and duration of mechanical ventilation. Patients with extubation failure had longer cardiovascular intensive care unit length of stay; however, the hospital length of stay was similar in both groups. The mortality both before hospital discharge and after hospital discharge was similar in the two groups.
Discussion
This study investigates incidence, aetiology, and outcomes of extubation failure in a homogeneous population of infants with shunt-dependent pulmonary blood flow and univentricular physiology. The incidence of extubation failure was high (∼27%) and aetiology diverse. Extubation failure in this study was not associated with increased hospital length of stay and mortality. However, there was a significant change in haemodynamics in children with extubation failure. There was a significant increase in heart rate, respiratory rate, and mean arterial pressure after extubation attempt in children with extubation failure. In addition, these patients also needed a higher fraction of inspired oxygen after extubation to maintain pre-extubation oxygen saturations and partial pressure of oxygen in arterial blood. The majority of extubation failures in this select patient population occurred in the first 24 hours.
In another study from our group, we demonstrated similar rates of extubation failure after the Norwood operation.Reference Gupta, McDonald and Gossett 16 Of the 64 eligible patients during the study period, extubation failure occurred in 22% (14/64) of the patients.Reference Gupta, McDonald and Gossett 16 However, extubation failure in infants after the Norwood operation was much slower, with 43% (6/14) of extubation failure occurring in the 24–96-hour period. This is in contrast to the current study where only 20% (3/15) extubation failure occurred in the 24–96-hour period. In another study by Harrison et al,Reference Harrison, Cox, Davis, Piedmonte, Drummond-Webb and Mee 3 lower reintubation rates (10%) after cardiac surgery have been reported. This difference in extubation failure rates between the two studies is related to the dissimilarity in study definitions and in the studied patient population. Harrison et al defined extubation failure as the need for reintubation within 24 hours of extubation and included patients ⩽36 months of age undergoing a variety of congenital cardiac surgery procedures. In contrast, we defined extubation failure as the need for reintubation within 96 hours and included infants <6 months of age. In our study, eight patients failed extubation in the first 24 hours, with an extubation failure rate of 15%. The rationale for using a 96-hour cut-off in our study was based on the reasoning that extubation failure in children with cardiac disease frequently evolves slowly and may often occur within 24 to 96 hours after extubation. In addition, a younger cohort with a more complex cardiac diagnosis in our study with a higher case mix index compared with those included by Harrison et al may also have contributed to higher reintubation rates in our patients.
Our study further demonstrated that the size of the Blalock–Taussig shunt was not associated with increased incidence of extubation failure. Blood flow between pulmonary and systemic circulations has to be optimally balanced before successful extubation in infants with shunt-dependent pulmonary blood flow. Inadequate pulmonary blood flow, due to inadequate shunt, pulmonary artery distortion, or pulmonary hypertension can cause severe hypoxaemia and extubation failure. On the other hand, increased pulmonary blood flow with elevated oxygen saturations can impair the ability to wean the child from mechanical ventilation. This may occur along with low cardiac output syndrome, with the use of larger shunts, and presence of residual aortic arch obstruction.
Our study demonstrated an increase in heart rate and respiratory rate after removal of positive pressure ventilation. There was also an increase in mean arterial blood pressure after removal of positive pressure ventilation. However, the increase in mean arterial blood pressure was more prominent in children with extubation success. This was associated with a decrease in inotrope score, suggesting improvement in cardiac output after transition to spontaneous breathing. Bronicki et alReference Bronicki, Herrera and Mink 17 demonstrated similar findings of improvement in mean arterial pressure after removal of positive pressure in children after repair of tetralogy of Fallot. The central venous pressures in our study remained unchanged after removal of positive pressure ventilation. This finding was similar to findings from Bronicki et alReference Bronicki, Herrera and Mink 17 where right atrial pressures did not change after extubation. Our study demonstrated that children with extubation failure needed higher fraction of inspired oxygen to maintain pre-extubation oxygen saturations and partial pressure of oxygen in arterial blood after removal from positive pressure when compared with the extubation success group. This finding suggested the presence of either lung collapse leading to dead space or problems with pulmonary shunt leading to ventilation–perfusion mismatch. In patients with shunt-dependent pulmonary blood flow, sudden removal of positive pressure ventilation can also lead to pulmonary oedema, thereby precipitating respiratory failure. Removal of positive pressure ventilation may dramatically worsen the ongoing low cardiac output state and precipitate sudden cardiac arrest.Reference Jenkins, Lynn, Edmonds and Barker 18 Furthermore, extubation-related stress response causes massive catecholamine surge that can initiate life-threatening arrhythmias or precipitate a pulmonary hypertensive crisis, thereby leading to sudden cardiovascular collapse.Reference Székely, Sápi, Király, Szatmári and Dinya 19
Optimal timing of extubation in children after cardiac surgery is based on clinical evaluation of the patient's ability to sustain spontaneous breathing without eliciting significant sympathetic response. Successful weaning from positive pressure ventilation depends on the presence of adequate cardiovascular function, satisfactory ventilatory reserves, and favourable pulmonary mechanics including functioning diaphragms. On the basis of the findings from this study, we recommend screening chest radiographs for atelectasis, pulmonary oedema, and diaphragmatic position before extubation in these patients. Before extubation, the patient should be assessed for the presence of inadequate cardiac reserves or low cardiac output syndrome using clinical surrogates such as trends in vital signs and physical examination, erosion of an excess of blood base, serum lactate levels, transcutaneous mixed saturations of oxygen as measured by near infrared spectroscopy, and invasive monitoring of arterial blood and central venous pressures. An important tool for measuring cardiac function before extubation can be an echocardiogram, especially in patients with marginal haemodynamics. Although respiratory muscle strength is not routinely assessed in these patients, commonly treatable disorders impairing strength and endurance such as hypokalemia, hypophosphatemia, and malnutrition should be addressed before extubation.
Our study has several limitations. This single-centre study generated results that may be unique to our institution and not generalisable to all centres. The retrospective nature of the study renders it susceptible to study design flaws and bias. We did not consider the role of weaning difficulties from mechanical ventilator in the group with failed extubation. Although we refer to the number of sedation and analgesic infusions and boluses, we also did not clarify on the dosages of sedation and analgesic infusion agents used in our cohort before removal of positive pressure ventilation. The number of patients with extubation failure was small, which limits our capability to precisely identify the predictors for extubation failure. To study the effect of extubation on haemodynamics, we had to rely on indirect markers of cardiac output such as mean arterial pressure, heart rate, oxygen saturation, near infrared spectroscopy, and lactate levels. It may therefore be prudent in future studies to have a direct measurement of cardiac output in these patients. Moreover, the predictors for extubation success should be rigorously evaluated in future, prospective, multi-centre trials.
In conclusion, this study demonstrates that extubation failure in patients with shunt-dependent pulmonary blood flow is high and that aetiology is diverse. Cardiopulmonary effects of removal of positive pressure ventilation are more pronounced in children with extubation failure and include escalation in the need for oxygen requirement and increase in mean arterial blood pressure. The majority of extubation failures in this select patient population occurs in the first 24 hours. Extubation failure in these patients is not associated with increased hospital length of stay or mortality.