Obstruction within the left ventricular outflow tract, when the ventriculo-arterial connections are concordant, produces an impediment to the flow of blood between the left ventricle and the aorta. When the ventriculo-arterial connections are discordant, it is the channel between the left ventricle and the pulmonary trunk which is impeded, but by the same individual obstructive lesions. Thus, the restriction to flow can be localized at valvar or subvalvar levels, with the potential substrates for stenosis being valvar, deviation of the muscular outlet septum, bulging of the septum, a fibrous shelf or fibromuscular tunnel, tissue tags, and anomalous attachment of the atrioventricular valvar tension apparatus (Fig. 1).

Figure 1 This cartoon shows the different lesions that can cause subpulmonary obstruction in the setting of discordant ventriculo-arterial connections. These lesions are exactly the same either in hearts with normal segmental connections, or in those with discordant ventriculo-arterial connections, albeit that they produce subaortic obstruction when the ventriculo-arterial connections are concordant.
There is a wide variety of approaches to alleviate obstruction within the left ventricular outflow tract when the ventriculo-arterial connections are discordant. Choosing between them involves not only knowledge of the different surgical techniques, but also understanding the particular anatomic features present in a specific patient. Our objective in producing this review is to examine all these surgical and anatomic aspects.
Concordant ventriculo-arterial connections
In the heart with normal segmental connections, several surgical procedures have been developed to overcome obstruction, and assure a better postoperative haemodynamic performance. Valvar lesions can be treated directly in some cases, with preservation of the previously stenotic valve. This is accomplished either by balloon, or by surgical valvoplasty.Reference Stark, de Leval, Tsang and Courtney1 In the first procedure, cardiac catheterization is required, and a deflated balloon is guided through the femoral artery and aorta until it reaches the aortic valve. The balloon is then inflated progressively, stretching the aortic valvar orifice to a reasonable size. The surgical approach to repairing the obstructive valve consists of separating the valvar leaflets by using appropriate incisions in the fused zones of apposition between the leaflets. Mobility of the leaflets, and the size of the effective valvar orifice, can be further improved by thinning out areas of thickenings or myxomatous nodules. In other instances, the valvar lesion may not be amenable to repair, and valvar replacement may be indicated. A prosthetic valve, an aortic homograft, or an autologous pulmonary valve can be used as substitutes. In the Ross operation, the pulmonary valve and its segment of supporting infundibulum are harvested along with part of the pulmonary trunk, and transferred to aortic position. A pulmonary homograft is later inserted in the pulmonary outflow tract. This technique has ensured that a valve made of “self tissue” will be supporting the systemic circulation. As a result, there is no need postoperatively for anticoagulation, and it can be expected that the valve will have considerable longevity, coupled with the potential for growth. All these advantages have made the Ross procedure an attractive solution for aortic valvar replacement, especially in young patients.
When the stenosis is subvalvar, it may be possible to relieve those obstructions which are discrete by direct resection through the aortic valve. In diffuse stenosis, or the tunnel variants with a small aortic root, a different approach is usually required.Reference Van Son, Schaff, Danielson, Hagler and Puga2 In this setting, the Konno-Rastan procedure is one possible solution.Reference Konno, Imai, Iida, Nakajima and Tatsuno3, Reference Rastan and Koncz4 The aortoventriculoplasty involves merging two longitudinal incisions, one into the aortic root, and the other into the muscular ventricular septum via the outflow tract of the right ventricle, thus providing access to the left ventricular outflow tract. The placement of a prosthetic patch onto the incised ventricular septum provides a larger left ventricular outflow, and a prosthetic or homograft valve is implanted in the newly enlarged aortic root. The Ross procedure has now been incorporated with this approach, giving the so-called Ross-Konno procedure.Reference Calhoon and Bolton5 It is also possible to modify the Konno operation should the aortic valve and the aorto-left ventricular junction be normal, thus preserving the native aortic valve.
Anatomic substracts of obstruction with discordant ventriculo-arterial connections
As emphasised above, the spectrum of lesions is exactly the same in hearts with normal segmental connection as in those with discordant ventriculo-arterial connections (Fig. 1). In the latter setting, usually known simply as “transposition”, obstructive lesions have been described in from one-eighth to onethird of cases,Reference Shrivastava, Tadavarthy, Fukuda and Edwards6–Reference Sansa, Tonkin, Bargeron and Elliott9 being more common in the presence of a ventricular septal defect than when the ventricular septum is intact.Reference Silberbach, Castro, Goldstein, Lucas and Edwards8
When stenosis is produced by a malformed pulmonary valve, the valve is usually bifoliate and dysplastic, albeit that stenosis at valvar level is often accompanied by other forms of subpulmonary stenosis (Fig. 2). The isolated form is relatively rare.Reference Shrivastava, Tadavarthy, Fukuda and Edwards6, Reference Elliott, Neufeld, Anderson, Adams and Edwards10, Reference Essed11 Malalignment of the outlet septum (Fig. 3) is among the most common conditions.Reference Essed11 The posterior and leftward deviation of the muscular outlet septum produces the barrier to the egress of blood from left ventricle. A septal bulge (Fig. 4) is often no more than the consequence of the disordered haemodynamics. The ventricular pressures in the right side, connected to the systemic circulation, are usually high. This is particularly true if the ventricular septum is intact, not allowing decompression between the right and left-sided cavities. As a result, there is a bowing of the septum towards left ventricle outflow. This dynamic type of obstruction can be further accentuated by thickening of the septal musculature.

Figure 2 In this heart with discordant ventriculo-arterial connections, shown from the left, there is obstruction to the left ventricular outflow tract caused by valvar stenosis together with anomalous insertion of tension apparatus of the mitral valve.

Figure 3 This heart with discordant ventriculo-arterial connections was photographed in anatomic orientation from the left. The posterior deviation of the outlet septum through the ventricular septal defect produces marked narrowing of the supulmonary outlet.

Figure 4 This parasternal long-axis view of a heart with concordant ventriculo-arterial connections shows a septal bulge causing subaortic obstruction. Exactly the same lesion can be responsible for subpulmonary obstruction in the setting of discordant ventriculo-arterial connections.
Tissue tags (Figs 5 and 6), and fibrous shelves (Figs 6 and 7), are both examples of fibrous subpulmonary stenosis. The tags represent tissue from an adjacent fibrous structure, usually an atrioventricular valve, and more often the tricuspid valve, herniating through an associated interventricular communication. The fibrous shelf can assume the shape of a diaphragm encircling outflow tract, or appear as a thickening extending sometimes from beneath the non-coronary leaflet of the aortic valve to the aortic leaflet of the mitral valve.

Figure 5 In this view of the left ventricular outflow tract in a specimen with discordant ventriculo-arterial connections, seen in anatomic orientation, there is obstruction due to a large tissue tag herniated from the undersurface of one of the leaflets of the tricuspid valve.

Figure 6 This view of the left ventricle from a specimen with discordant ventriculo-arterial connections, seen in anatomic orientation from the left, shows an extensive fibrous shelf that continues on to the pulmonary leaflet of mitral valve. There are also tissue tags, herniated from the leaflets of the mitral valve, which further obstruct the outflow tract.

Figure 7 This view of the left ventricle from a specimen with discordant ventriculo-arterial connections, seen from the left, shows an extensive fibrous shelf, which again continues to involve the pulmonary leaflet of the mitral valve.
Another cause of fixed obstruction is the subvalvar fibromuscular tunnel (Fig. 8), which is often associated with a hypoplastic pulmonary valve. Anomalous attachment of the cords from the left atrioventricular valve (Fig. 2) can also restrain the passage of blood. Other anomalies, such as a cleft anterior leaflet or mitral valvar rotation, may also be found.

Figure 8 This heart with discordant ventriculo–arterial connections was sectioned across the outflow tracts to show a subpulmonary infundibulum, along with a squeeze between the outlet septum (OS) and the left ventricular wall. In essence, the combination produces tunnel-like stenosis, albeit muscular in this instance.
Surgical procedures
In the early 1960s, the interatrial switch (Fig. 9), performed by either a Mustard or Senning procedure, was the most popular method for definitive repair of transposition. Blood coming from caval veins was redirected at atrial level to the left ventricle. In the same way, blood arriving from pulmonary veins was delivered to the right-sided cavities. When there was obstruction of the left ventricular outflow tract, the technique was often complemented by seeking directly to resect the stenosis. Residual obstruction, however, was known to court the risk of exaggerating the high systemic venous pressure. In addition, the atrial procedure itself produced a number of early and late complications, including disruption of sinus rhythm as a result of damage to the sinus node or its blood supply, supraventricular arrhythmias, sudden death, decompensation of right ventricular function, and obstruction to venous return, either systemic or pulmonary. Use of the atrial procedure, combined with insertion of a conduit from the apex of the left ventricle to the pulmonary arteries, nonetheless, did produce good results in some hands,Reference Crupi, Anderson, Ho, Lincoln and Buckley12 and was combined with reasonable outcome in the medium term.Reference Crupi, Pillai, Parenzan and Lincoln13

Figure 9 This cartoon shows the common principle subjacent to Mustard and Senning procedures. (a) shows in diagrammatic fashion, the redirection of pulmonary and systemic venous flows at atrial level. An external conduit (b) is placed to overcome the obstruction within the left ventricular outflow tract.
In an attempt to find a better solution for surgical repair, Rastelli and his colleagues in 1969,Reference Rastelli, McGoon and Wallace14 proposed the creation of an intraventricular tunnel from left ventricle to aorta, passing through the interventricular communication (Fig. 10). The procedure, of necessity, was limited to those patients that had a suitable ventricular septal defect. The new communication between the right ventricle and the pulmonary arteries was re-established using an extracardiac conduit. This represented a major advance, since it restored the morphologically left ventricle to the role of supporting the systemic circulation. Long-term survival, however, has proved unsatisfactory.Reference Dearani, Danielson, Puga, Mair and Schleck15, Reference Kreutzer, De Vive and Oppido16 The complications are related mainly to the need of an extracardiac conduit, specifically the risk of this substernal structure having to be replaced if it becomes obstructive, and late recurring obstruction within the left ventricular outflow tract.

Figure 10 The cartoon shows the main steps of Rastelli procedure in the setting of a deviated outlet septum (a). A patch is placed to create an interventricular tunnel (b), and an extracardiac conduit is placed between the right ventricle and the pulmonary arteries (c).
Perhaps the most important step forward in surgical treatment of patients with transposition was achieved by Jatene in 1975,Reference Jatene, Fontes, Paulista, Souza, Neger, Galantier and Sousa17 when he performed the first successful arterial switch operation (Fig. 11) The concept of dividing the aorta and the pulmonary trunk, transposing their distal connections, and transferring the coronary arteries to the new aortic root, had long been suggested as the optimal treatment for discordant ventriculo-arterial connections, but success had been elusive until the landmark achievement of Jatene. The procedure resulted in fewer short and long-term complications, as it provided a more anatomical and physiological means of correction. One of the contraindications for the arterial switch procedure, nonetheless, was recognised to be obstruction to left ventricle outflow tract, so this spawned other innovative surgical procedures.

Figure 11 The cartoon illustrates the arterial switch procedure. It shows the connection of the proximal great arteries to the distal end of the other great artery, as well as the transfer of coronary arteries to the new aorta.
Thus, a suggested improvement to the approach recommended by Rastelli and his colleagues was the “reparation a l’etage ventriculaire”, or REV procedure (Fig. 12), initially described by LecompteReference Lecompte, Neveux and Leca18 in 1982. As with the Rastelli approach, the manoeuvre can only be used in presence of ventricular septal defect, but it involved two major differences. First, the muscular outlet septum was extensively resected, providing a better pathway between the aorta and the left ventricle. As a result, the intraventricular tunnel created was shorter, and was alleged to produce a better haemodynamic platform. Secondly, the combined use of the Lecompte manoeuvre, bringing the bifurcation of the pulmonary trunk anterior to the aorta, made it possible to re-implant the valveless pulmonary trunk directly to the right ventricle, thus avoiding the use of an extracardiac conduit. Another potential advantage was the possibility of performing the operation during infancy, in this way avoiding the attritions from initial palliations. This option of early repair is not possible with the Rastelli approach, since the fate of small extracardiac protheses remains unfavourable. Studies comparing the two techniques have shown similar early and late mortalities, but a lower likelihood of reoperation and residual obstruction with reparation a l′etage ventriculaire.Reference Vouhé, Tamisier, Leca, Ouaknine, Vernant and Neveux19, Reference Lee, Lim and Kim20
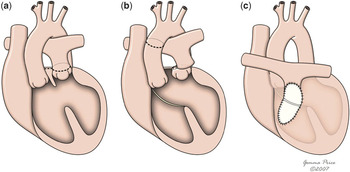
Figure 12 This cartoon shows the innovations of the REV procedure relative to the Rastelli procedure, with resection of the muscular outlet septum (b) and use of Lecompte manoeuvre (c) which avoids the use of an extacardiac conduit.
An aortic translocation approach that has recently come to the fore was introduced by Nikaidoh,Reference Nikaidoh21 in 1984, who himself revisited the concept initially proposed by BexReference Bex, Lecompte, Baillot and Hazan22 for anatomical repair achieved by resecting the aortic infundibulum (Fig. 13). Instead of using distal aortic transection and coronary arterial transfer, as was the case with the arterial switch, Bex had removed the aorta together with its valve and a rim of subvalvar infundibular muscle. He had then translocated the entire root to the usually posterior pulmonary position. This technique avoided transfer of the coronary arteries, and could be done with an intact ventricular septum, as in the initial description by Bex.Reference Bex, Lecompte, Baillot and Hazan22 The modification proposed by NikaidohReference Nikaidoh21 also commenced with aortic translocation, but in association with reconstruction of the outflow tracts of both ventricles. This modification required the ventricular septum to be deficient. The aortic root is harvested with the coronary arteries attached, the muscular outlet septum is bisected, and the pulmonary valve is excised, thus relieving obstruction of the left ventricular outflow. The posterior part of the aortic root is sutured to the pulmonary root, but the anterior rim is supported by the patch used to close the ventricular septal defect. At this point, the Lecompte manoeuvre, bringing forward the bifurcation of the pulmonary trunk, can be performed to preclude stenosis of the right pulmonary artery. If the pulmonary trunk itself is hypoplastic, it is opened longitudinally. The right ventricular outflow tract is then reconstructed using a pericardial patch. The operation is technically challenging, with potential interference of the course of the coronary arteries, especially in the setting of unconventional patterns. Furthermore, the need of reoperation, as a result of either right ventricular outflow tract obstruction or pulmonary insufficiency, has been reported. There are several positive aspects. First, it avoids the need for an extracardiac conduit, minimising early reoperations. Secondly, it relieves the outflow obstruction and provides a more direct passage from the left ventricle to the aorta, thus permitting the insertion of a smaller interventricular patch without major interference of the left ventricular integrity.

Figure 13 The cartoon shows the major issues of Nikaidoh’s procedure. (a) shows the areas for harvesting the aortic root and transection of the pulmonary trunk. In (b), the aorta and pulmonary trunk have already been disconnected from their previous insertion, and the line of division of the outlet septum is shown. (c and d) show aortic translocation and reconstruction of the left outflow tract, with a pericardial patch used to close the ventricular septal defect. In (e) and (f), the pulmonary trunk is sutured to the right ventricular outflow tract, and the reconstruction is completed with a pericardial patch.
A further modification of reparation a l’etage ventriculaire was proposed by Metras and colleaguesReference Metras, Kreitmann and Riberi23 in 1997. These authors argued against the use of the Lecompte manoeuvre, and instead made the connection between the pulmonary trunk and the right ventricle inserting a segment of aortic autograft, with or without an additional monocusp patch. According to Metras and colleagues,Reference Metras, Kreitmann and Riberi23 this improved the results, and permitted normal growth of the autograft.
Choosing the most appropriate surgical procedure
Several aspects need to be considered when deciding the best approach to the surgical correction of discordant ventriculo-arterial connections in the setting of an obstructed left ventricular outflow tract. Clinical issues, such as age, weight, and severity of symptoms, as well as morphological and echocardiographic data, the experience of the surgeon, early mortality, long-term outcome, and complications inherent to each technique are all pertinent in deciding the most appropriate type of intervention, and the best time for doing it.
Nowadays, the arterial switch has become the procedure of choice for repair of most forms of discordant ventriculo-arterial connections. Late outcomes, as presented by Losay et al.Reference Losay, Touchot and Serraf24 in a large population of patients, have been very encouraging. Long-term mortality is low, left ventricular function is usually good, and sinus rhythm is generally preserved. Complications, like aortic insufficiency and coronary arterial obstruction, have been rare, and reoperation has been needed in one-tenth of cases, mainly due to pulmonary stenosis.
In the particular setting of an obstructed left ventricular outflow, the arterial switch seems to retain its primacy in a selected subset of patients.Reference Karl, Cochrane and Brizard25, Reference Sohn, Brizard, Cochrane, Wilkinson, Mas and Karl26 Thus, use of the switch, combined with primary resection of the obstructive lesions, remains applicable to dynamic forms of obstruction, and to some fixed resectable obstacles, when there is a good sized ventriculo-arterial junction. In this respect, the gradient between the left ventricle and the pulmonary trunk is not itself a reliable predictor of the severity of stenosis and the post-operative outcome. The gradient can be overestimated as a result of high pulmonary flow, a common finding in transposition. Instead, attention should be focused on morphological criterions.Reference Sohn, Brizard, Cochrane, Wilkinson, Mas and Karl26, Reference Wernovsky, Jonas and Colan27
Anomalous pulmonary valves can be repaired using the same techniques as employed in the setting of aortic stenosis. If a good relief of obstruction is achieved, the arterial switch can then safely be accomplished.Reference Uemura, Yagihara and Kawashima28 In the same way, tissue tags and fibrous shelves can usually be resected, so patients with these lesions are good candidates for this kind of procedure. Dynamic obstructions revert after surgery because of the considerable reduction of septal bulge in consequence of the reversal of the pressures in the morphologically left and right ventricles.
Obstructions caused by marked deviation of outlet septum, or presence of fibromuscular tunnel, are more difficult to manage, taking into consideration of the proximity of the mitral valve and the conduction tissues. To avoid these important structures, muscular excision should be restricted to the area between eleven and two o’clock as seen from the perspective of the surgeon.Reference Stark, de Leval, Tsang and Courtney1 In many cases, removal of this limited area is sufficient to achieve good patency, making it possible then to proceed with an arterial switch. Accessory attachments of the mitral valvar leaflets can be divided. Malattachment of the ‘proper’ tension apparatus of the mitral valve across the left ventricular outflow tract, however, is a relative contra-indication to the arterial switch procedure.
Intact ventricular septum
In those cases where the arterial switch procedure does not offer an unobstructed, or minimally obstructed, connection between the left ventricle and the neo-aorta, other alternatives for anatomical correction must be considered. The options very much depend on the presence or absence of an interventricular communication (Fig. 14). Should the ventricular septum be intact, an atrial switch combined with insertion of a conduit from the left ventricle to the pulmonary arteries may still be the only way of providing palliative repair.

Figure 14 This scheme illustrates how morphological aspects may have influence in the choice of the most appropriate surgical procedure in the setting of discordant ventriculo-arterial connections with left ventricular outflow obstruction (TGA+LVOTO). We consider the presence of a minor straddling valve, a severely dysplastic or hypoplastic pulmonary valve, a small right ventricle, a fibromuscular tunnel unressectable through the pulmonary valve, and an inlet or restrictive septal defect, all as evidence of unfavourable anatomy.
Presence of a ventricular septal defect
If a septal defect is present, the obvious options from which to choose are a Nikaidoh procedure, a REV procedure or its modification, or the Rastelli operation. Traditionally, the Rastelli procedure was the preferred option. Long-term results reported by Boston Children’s Hospital and Mayo Clinic, however, have been disappointing.Reference Dearani, Danielson, Puga, Mair and Schleck15, Reference Kreutzer, De Vive and Oppido16 Furthermore, the operation is inappropriate in presence of certain unfavourable intracardiac anatomic features. A small right ventricle contraindicates the procedure, since the intraventricular tunnel would further reduce its dimensions. It is also difficult to construct the connection from left ventricle to aorta through a small and restrictive ventricular septal defect, and this would require laterosuperior enlargement of the defect.Reference Stark, de Leval, Tsang and Courtney1 The defect needs to be subaortic, and not in a remote position. Multiple defects also do not lend themselves to this type of reconstruction. Besides the configuration of the septal defect, valvar morphology should also be taken into consideration. Presence of a straddling mitral valve has proven to be a risk factor for early and late death.Reference Kreutzer, De Vive and Oppido16 In addition, abnormal attachments of the tension apparatus of the tricuspid valve to the outlet septum may also pose problems for the placement of the baffle between the left ventricle and the aorta. The course of the coronary arteries should be determined in order to ensure that a major coronary arterial branch does not cross the right ventricular outflow tract, as this would make right ventriculotomy undesirable.
In the REV operation, many of these contraindications remain, such as a remote or multiple defect, ventricular hypoplasia, and potential problems with the coronary arteries. Moreover, certain conditions such as hypoplasia or stenosis of the pulmonary arteries, elevated pulmonary vascular resistance, and pulmonary arterial distortion should be added to the list.Reference Lee, Lim and Kim20 This is due to the tendency of patients to develop obstruction of the right ventricular outflow tract after the procedure, as a result of the stretching produced by the anterior location of the pulmonary arteries.Reference Vouhé, Tamisier, Leca, Ouaknine, Vernant and Neveux19 The applicability of the procedure in the setting of a small septal defect, and in presence of abnormal attachments of the tricuspid valve to the outlet septum, nonetheless, gives it other advantages in relation to the Rastelli procedure.
The approach recommended by Nikaidoh provides a possible solution in some difficult anatomic contexts not amenable to either the REV or Rastelli approaches. There are now several reports in the literature of successful correction in challenging settings.Reference Kandeel, Kumar, Prabhakar, Halees and Duran29, Reference Morell and Wearden30 The exposure provided after initial removal of the aortic root allows a good visualization of both the interventricular defect and the atrioventricular valves. This allows closure of the septal defect when there is minor straddling of an atrioventricular valve. It is also particularly suitable should it not be possible to resect stenosis caused by a fibromuscular tunnel, or by a severely dysplastic or hypoplastic pulmonary valve. A restrictive or inlet septal defect, right ventricle hypoplasia, or an aberrant coronary artery, are also not limiting factors. The mid-term follow-up has shown that the Nikaidoh approach has better results when compared to the Rastelli operation.Reference Yeh, Ramaciotti, Leonard, Roy and Nikaidoh31, Reference Bautista-Hernandez, Marx, Bacha and del Nido32 Further studies in larger series, nonetheless, are needed to confirm its routine applicability.
Other morphological aspects to be taken into consideration
Coronary arterial pattern
A wide variety of coronary arterial origins and courses are known to exist when the ventriculo-arterial connections are discordant. Their description is particular important when planning any surgical procedure, and to foresee any potential technical problems in coronary transfer.
Several attempts have been made to classify the coronary arterial anatomy. The first was suggested by Yacoub and Radley-SmithReference Yacoub and Radley-Smith33 in 1978. They gave special focus to the presence of single orifice, close origin of an arterial orifice near to the facing commissure, the circumflex artery arising from the right coronary artery and crossing behind the pulmonary trunk, and the circumflex artery originating from the posterior “right” sinus. The disadvantage of their alphabetic classification, however, became apparent as new patterns were discovered.
Five years later, the group from Leiden UniversityReference Gittenberger de Groot, Sauer, Oppenheimer-Dekker and Quaegebeur34 proposed that the aortic sinuses adjacent to the pulmonary trunk should be named “sinus 1” and “sinus 2”. Sinus 1 was positioned to the right hand of an observer standing in the non-adjacent sinus, looking towards the pulmonary trunk. Sinus 2 was to the left hand. They then pointed out that, since there were only three main arteries, which without exception arose from one or other adjacent sinuses, all patterns could be taken into account simply by combining these two features. The simplicity of this nomenclature has now made it the preferred means of describing arterial origins.Reference Lacour-Gayet and Anderson35
During the last decade, several studies have shown a correlation between an increased risk of post-operative events and certain coronary arterial patterns, such as a single coronary arterial orifice, or intramural or looping courses.Reference Legendre, Losay and Touchot-Koné36–Reference Bonhoeffer, Bonnet and Piéchaud39 Not all, however, share these opinions.Reference Hutter, Bennink, Ay, Raes, Hitchcock and Meijboom40, Reference Scheule, Zurakowski and Blume41 Whether a difficult coronary arterial arrangement can or cannot be a contraindication to the arterial switch procedure is still a matter of discussion.
It is generally accepted, nonetheless, that the development of more suitable techniques for coronary arterial transfer has simplified the arterial switch procedure in complicated situations. A recent meta-analysis of nine independent seriesReference Pasquali, Hasselblad, Li, Kong and Sanders42 concluded that certain rare coronary arterial patterns, including those with an intramural or single coronary artery, produced a significantly increased risk of mortality, which was maintained over time. Moreover, a recent multi-institutional study from the European Congenital Heart Surgeons AssociationReference Sarris, Chatzis and Giannopoulos43 showed, in univariate analysis, that coronary arterial anomalies are associated with increased mortality.
Other aspects of the coronary circulation should also be taken in account, as they can create other technical difficulties. Malalignment of facing commissures, and early branching of the coronary arteries, particularly the artery to the sinus node, may further complicate the procedure, and their presence should always be assessed.Reference Massoudy, Baltalarli and de Leval44
Conduction system
As we have previously described, it is sometimes possible to resect obstructive lesions through the pulmonary valve. In this situation, the surgeon must take great care to avoid the conduction tissues. In discordant ventriculo-arterial with concordant atrioventricular connections, the atrioventricular conduction axis is usually situated on the left side of the ventricular septum, in subendocardial position related closely to the superficial fibromuscular tissue.Reference Crupi, Anderson, Ho, Lincoln and Buckley12 This feature can limit removal of the obstructive lesions, and hence similarly limit the applicability of an arterial switch procedure. As far as the Nikaidoh procedure is concerned, however, the location of the conduction system does not in itself pose any technical obstacle to its execution, since the outlet septum can be divided and sutured without putting in danger the atrioventricular conduction axis. It is an unviolable rule that the muscular outlet septum never contains conduction tissues.
Conclusion
Obstruction to the left ventricular outflow tract is a major complication in the setting of discordant ventriculo-arterial connections, the treatment of which has evolved markedly in the last decades. Biventricular reconstruction in this setting can still represent a great surgical challenge. The arterial switch is now usually considered the first option, but its feasibility needs to be assessed after evaluation of the coronary arterial patterns, along with the anatomic characteristics of the obstructed outflow tract. Special attention should be given to the size of ventriculoarterial junction, the nature of the obstacle, the severity of stenosis, and its potential resectability. In our unit, we would prefer to achieve a less than perfect relief of the left ventricular obstruction, rather than place a conduit for the Rastelli procedure.
If an arterial switch proves not to be possible, it is morphological features which again assume an important role in choosing between the other possible approaches. A profound knowledge of the individual anatomy is then a fundamental requisite in surgical planning, as it contributes not only to a correct choice of surgical technique, but also to the foreseeing of difficult situations, and the consequent avoidance of post-operative complications.
Acknowledgements
During this investigation, Dr Martins was supported by the Portuguese Society of Cardiology and by the Calouste Gulbenkian Foundation.
Andrew Cook, Vi Tran, and Gemma Price are supported by the British Heart Foundation. Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from R&D funding received from the NHS Executive.
















