Case report
A 26-year-old female patient was referred to our centre with congestive heart failure (CHF). She had a history of hypothyreodism, adipositas and thrombophlebitis. In addition, she had a psychiatric disorder and received quetiapin.
On admission she presented with acute distress with tachydyspnea at rest and tibial edema. Symptoms had started after an infection of the upper airways, which had been treated with amoxicillin. Surface ECG demonstrated atrial fibrillation and repolarisation abnormalities. Echocardiography showed markedly reduced left ventricular ejection fraction (LVEF 20%) and dilatation of all cardiac chambers. Atrial fibrillation was terminated by DC cardioversion. Apixaban was started for the prevention of thrombosis. Endomyocardial biopsies demonstrated acute myocarditis with a high Parvovirus B19 virus load, and left ventricular end-diastolic diastolic pressure (LVEDP) was 12 mmHg. Cardiac function improved after initiation of hydrochlorothiazide, spironolactone, bisoprolol, ramipril (5 mg/d) and a 24-hour infusion of levosimendan.
Shortly after starting anticongestive medication, the patient started to expectorate bronchial casts with varying frequencies (three times per week to five times daily; Fig 1). Pneumologic workup, including histology of the casts, microbiology, and a CT scan of the lungs, did not reveal any cause for bronchial cast formation. Inhaled corticosteroids were started without any benefit regarding expectoration of bronchial casts.
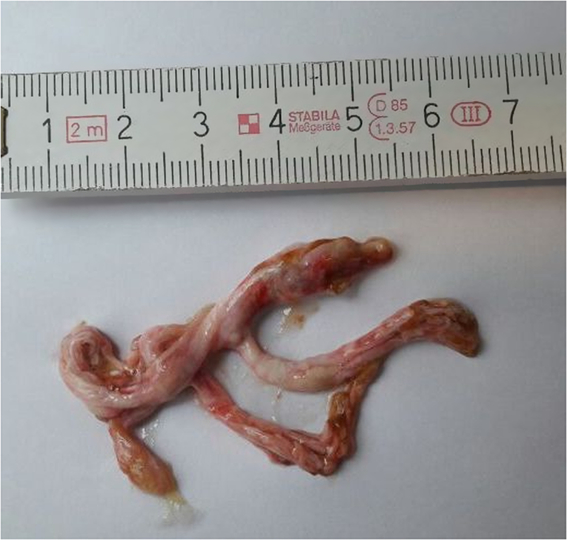
Figure 1. Expectorated bronchial cast of our patient.
Two years later, re-evaluation of haemodynamics revealed a persistingly elevetated LVEDP of 14 mmHg. Repeat endomyocardial biopsies were negative for virus genome but revealed mild myocardial fibrosis resulting from former myocarditis.
Finally, afterload reduction therapy was switched from ramipril to candesartan in an attempt to reduce cast formation. Expectoration of bronchial casts stopped within 24 hours thereafter. At that time no other modification of the medical regimen had been performed. Four weeks later, re-exposition to ramipril prompted immediate re-appearance of cast formation, which again immediately stopped after switching back to candesartan.
Discussion
Bronchial casts are mucoid, gelantinous formations in the airways consisting of bronchial mucus. Symptoms vary from cough to potentially life-threating suffocation. Reference Schumacher, Singh, Kuebler, Aprile, O’Brien and Blume1
Generally, bronchial cast formation has been described in patients with congenital heart disease (CHD) or pulmonary disease. Reference Kunder, Kunder and Sun2 Among patients with CHD, those with Fontan circulation have a particularly high risk for formation of bronchial casts with a reported incidence of 1–4%. Reference Kunder, Kunder and Sun2 In addition, bronchial casts can result from severely impaired haemodynamics after surgery for CHD. Reference Avitabile, Goldberg, Dodds, Dori, Ravishankar and Rychik3 Asthma bronchiale, tuberculosis and cystic fibrosis are the main pulmonary diseases associated with plastic bronchitis. Reference Avitabile, Goldberg, Dodds, Dori, Ravishankar and Rychik3 Furthermore, it is assumed that noxious agents such as smoke are risk factors for the development of plastic bronchitis. Reference Schmitz, Schatz and Kirsten4
Therapeutic attempts to control bronchial cast formation have been performed with medical therapy, catheter interventions and surgery. Therapeutic agents included inhaled fibrinolytica, inhaled mucolytika, inhaled or systemic corticoisteroids or systemic immunsuppressants with reduction of symptoms in only few patients. Reference Brooks, Caruthers, Schumacher and Stringer5 To improve haemodynamics, sildenafil or bosentan as well as interventional methods or repeat surgery have been employed in patients after corrective surgery of CHD. Reference Singhi, Vinoth, Kuruvilla and Sivakumar6 However, our patient had elevated filling pressures (LVEP, CVP) as known risk factors for bronchial cast formation. There was no hint for an inflammatory process as an underlining cause.
In conclusion, we were able to identify ramipril as one cause for bronchial cast formation. This side effect has not been described yet for ramipril as well as any other angiotensine-converting enzyme (ACE) inhibitor.
The underlying pharmacological mechanism is unclear. We speculate that cast formation was related to the well-described activation of bradykinin by ACE inhibitors. Bradykinin induces microvasculare leakage at all airway levels. Due to its vasodilative action, bradykinin increases blood flow to the airways. Thus, it has a stimulatory effect on airway mucus secretion, which is the main component of bronchial cast formations. Reference Barnes, Chung and Page7 Furthermore, bradykinin itself stimulates C-fiber nociceptors, which causes chronic dry cough. Reference Abraham, Scuri and Farmer8 In our patient, however, cough was only present in cast expectoration.
Conclusion
Bronchial cast formation is a potentially life-threatening complication often associated with complex CHD. In our patient, cast formation occurred in the absence of CHD or any other cause known so far. To the best of our knowledge, this is the first report of bronchial cast formation due to ramipril treatment. Our case report may encourage colleagues involved in care of patients with bronchial cast formation to focus on ramipril or other ACE inhibitors as part of their therapeutic regimen, as cast formation may stop after switching to an alternative treatment. Further studies may elucidate possible pharmacological mechanisms resulting in bronchial cast formation.
Conflicts of Interest
All authors declare no conflict of interest (K. S. P., T. P., M. S.).
Ethical Standards
The patient gave written informed consent for publication of this report.



