Kawasaki disease is one of the most frequent vasculitides in children.Reference Barut, Sahin and Kasapcopur 1 Although its aetiology is unknown, Kawasaki disease is an acute and self-limiting, medium-sized vessel vasculitis.Reference Kawasaki 2 Approximately 25% of patients with Kawasaki disease develop coronary artery aneurysms if they are not treated with high-dose intravenous immunoglobulin during the early stages of the disease. Coronary artery involvement can lead to myocardial ischaemia, infarction, and sudden death.Reference Gordon, Kahn and Burns 3 In addition, myocardial fibrosis, valvulitis,Reference Nakamura, Yashiro and Uehara 4 and aortic root dilatationReference Ravekes, Colan and Gauvreau 5 can lead to cardiovascular complications in Kawasaki disease.
There are limited data available regarding cardiac function after the acute phase of Kawasaki disease. Subclinical dysfunction can be missed when assessing left ventricular systolic functions using conventional echocardiography. Myocardial deformation measurement is an effective method for determining cardiac function.Reference Gorcsan and Tanaka 6 Speckle-tracking echocardiography is a recently developed technique for evaluating myocardial deformation or strain when investigating cardiac function.Reference Mor-Avi, Lang and Badano 7 There are a few reports regarding the assessment of left ventricular myocardial deformation in children with Kawasaki diseaseReference Yu, Choi and Kim 8 , Reference Xu, Ding and Lv 9 , whereas left ventricular myocardial deformation during follow-up has not been investigated. Our objective, therefore, was to examine cardiac function using speckle-tracking echocardiography during mid-term follow-up of patients with antecedent Kawasaki disease.
Materials and methods
Patients
Patients who had Kawasaki disease during childhood and were followed-up at the Paediatric Rheumatology and Cardiology outpatient clinic of the Cerrahpasa Medical Faculty in Istanbul, Turkey, between January, 2002 and August, 2015, were recruited. They were considered as patients with antecedent Kawasaki disease and evaluated for their cardiac function. All patients fulfilled diagnostic criteria for Kawasaki diseaseReference Newburger, Takahashi and Gerber 10 ; furthermore, all the patients had undergone an initial echocardiographic examination before the intravenous immunoglobulin infusion therapy during the acute phase of the disease. Patients who had subsequently developed recurrent febrile episodes within months following the initial Kawasaki disease diagnosis were excluded from the study. During the follow-up, speckle-tracking echocardiography examinations had been performed at least 6 months after the acute phase of Kawasaki disease. The identified patient medical records, including age at diagnosis, duration of the disease, therapy, and clinical records, were evaluated. Laboratory data measured during the acute phase of the disease were obtained from medical records, specifically alanine transaminase, serum electrolytes such as sodium and potassium, serum albumin, C-reactive protein, white blood cell count, platelet count, haemoglobin levels, and presence of pyuria – that is, urinary white blood cell count >10 cells/high-power field.
Transthoracic echocardiography was performed at the Paediatric Cardiology Department of the Cerrahpasa Medical Faculty, using a commercially available echocardiography machine (Philips iE 33; Philips Medical System, Bothell, Washington, United States of America) equipped with a broadband X5S MHz transducer. Patients were grouped according to the presence of persistent aneurysm(s) following Kawasaki disease, either with or without coronary aneurysms. Persistent coronary aneurysm was detected in seven patients: three patients had a left anterior descending artery aneurysm, two had a right coronary artery aneurysm, and two patients had both left anterior descending artery and right coronary artery aneurysms.
We assessed the regional myocardial strain using speckle-tracking echocardiography in 35 asymptomatic patients with antecedent Kawasaki disease. The control group comprised 30, healthy, age- and sex-matched children referred to paediatric polyclinics for simple reasons such as an innocent heart murmur without cardiac disease having been found using physical and echocardiographic examinations. All patients and their families were informed, a written informed consent was obtained, and the study was approved by the ethics committee of the institution.
Strain imaging for myocardial deformation using speckle-tracking echocardiography
Speckle-tracking echocardiography is a new technique that analyses motion by tracking natural acoustic reflections within an ultrasonic window. “Speckles” are stable patterns composed of 20–40 pixels that are automatically tracked during the cardiac cycle to follow the myocardial motion and directly assess the ventricular deformation at regions of interest.Reference Kaluzynski, Chen and Emelianov 11
Longitudinal left ventricular mechanics are the most sensitive components of the left ventricular dynamics, and these components are most sensitive to the presence of myocardial disease.Reference Reisner, Lysyansky and Agmon 12 Therefore, we measured global longitudinal strain and circumferential strain using speckle-tracking echocardiography from six ultrasonic views. We acquired three consecutive beats using high frame-rate harmonic imaging in each echocardiographic view. Cardiac cycles were recorded as two-dimensional colour video loops, and the acquired raw data were saved for offline analysis (Xcelera and QLab; Philips Medical Systems) (Fig 1). Manual adjustment of the regions of interest was performed as necessary. The average of each measurement was calculated.
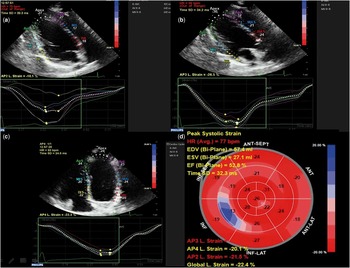
Figure 1 Automatically generated left ventricular deformation curves by the software (QLab). ( a ) Left ventricular longitudinal strain in the two-chamber view. ( b ) left ventricular longitudinal strain in the three-chamber view. ( c ) left ventricular longitudinal strain in the four-chamber view. ( d ) “Bulls Eye” schematic presentation of global strain measurements in left ventricular segments.
Statistical analysis
All variables were tested using the Kolmogorov–Smirnov test for normality. Normally distributed continuous variables of data are expressed as mean±standard deviation. Categorical data are expressed as percentages. Differences between the groups were assessed using independent sample t-tests for normally distributed variables. Chi-square or Fisher’s exact tests were used to analyse categorical data and proportions. The Pearson correlation was used to assess the correlation between different variables. A significance value of p⩽0.05 was used in all tests. All statistical procedures were performed using SPSS version 22 for Windows (SPSS Inc., Chicago, Illionois, United States of America).
Results
The demographic and conventional echocardiographic characteristics of patients with antecedent Kawasaki disease are summarised in Table 1. Their mean age at diagnosis was 25.6±15.4 months, and the median follow-up time was 57.5 months (16.5–98.2). No statistically significant differences were found between patients with antecedent Kawasaki disease and controls with respect to sex, age or systolic/diastolic indices, as measured on conventional echocardiography. Table 2 represents the initial laboratory and conventional echocardiographic features of patients with antecedent Kawasaki disease during the acute phase of the disease.
Table 1 Demographic and conventional echocardiographic characteristics of patients with antecedent Kawasaki disease and controls during analysis.

A=peak velocity of diastolic atrial mitral inflow; E=peak early diastolic velocity of mitral inflow; LVEDD=left ventricular end dimension; LVESD=left ventricular end-systolic dimension; LVPWT=left ventricular posterior wall thickness; LVSWT=left ventricular septal wall thickness
Data expressed as mean±SD
* The follow-up time was represented with median and quartiles in parenthesis
Table 2 Laboratory and conventional echocardiographic data of the antecedent Kawasaki disease patients during acute phase of the disease.

A=peak velocity of diastolic atrial mitral inflow; ALT=alanine amino transferase; CRP=C-reactive protein; Date=mean period elapsed between the beginning of fever and the beginning of intravenous immungloubuline therapy; E=peak early diastolic velocity of mitral inflow; Hgb=haemoglobin; LV=left ventricular; LVEDD=left ventricular end-diastolic dimension; LVESD=left ventricular end-systolic dimension; LVPWT=left ventricular posterior wall thickness; LVSWT=left ventricular septal wall thickness; n=number of patients, values are means±standard deviations; Wbc=white blood cells
* In normal ranges
Comparison of laboratory markers and left ventricular strain in patients with antecedent Kawasaki disease, with and without coronary aneurysm
A total of seven patients with persistent coronary aneurysm were maintained on long-term aspirin therapy. Among them, two patients had left coronary artery aneurysms of 5 and 3 mm in diameter, respectively; one patient had a giant aneurysm of 9 mm at the time of testing and was on warfarin therapy; two patients had right coronary aneurysms of 4.7 and 3.1 mm, respectively; and two patients had both right and left coronary aneurysms – one had a 4.6-mm aneurysm on the right coronary artery and a 4.3-mm aneurysm on the left coronary artery, and the other had an aneurysm of 7.2 mm on the left coronary artery and an aneurysm of 5.8 mm on the right coronary artery at the time of testing. Detailed information about patients with coronary aneurysms is presented in the Supplementary Table.
Patients with coronary aneurysms had higher C-reactive protein levels, heart rates, and systolic and diastolic blood pressures during the acute phase of antecedent Kawasaki disease compared with those without coronary aneurysms (p⩽0.05) (Table 3). Although fractional shortening was significantly lower in patients with coronary aneurysms than in those without coronary aneurysms (p⩽0.05), the ranges were within normal limits for systolic function in both groups. In addition, we compared controls with patients without coronary aneurysms and found no difference regarding their conventional echocardiographic results (Table 4).
Table 3 Clinical and laboratory data of antecedent Kawasaki patients with and without coronary aneurysms during the acute phase of the disease.

ALT=alanine amino transferase; CRP=C-reactive protein; Hgb=haemoglobin; n=number of patients; WBC=white blood cells
Table 4 Conventional echocardiographic data of participants

A=peak velocity of diastolic atrial mitral inflow; E=peak early diastolic velocity of mitral inflow; LV=left ventricular; LVEDD=left ventricular end-diastolic dimension; LVESD=left ventricular end-systolic dimension; LVPWT=left ventricular speckle-tracking echocardiography posterior wall thickness; LVSWT=left ventricular septal wall thickness
Values are means±standard deviations. Analyses were carried out among patients with coronary aneurysms and without coronary aneurysms and also amongpatients without coronary aneurysms and controls. Unless noted by α, the p value was insignificant.
* Reference values were obtained from Eidem et alReference Eidem, McMahon and Cohen 34
There was no significant difference in strain values between antecedent Kawasaki disease patients with and without coronary aneurysms in left ventricular segments (p>0.05) (Table 5).
Table 5 Strain measurements with speckle-tracking echocardiography data of patients with antecedent Kawasaki disease and controls.

AHA=American Heart Association; EDV=end-diastolic volume; ESV=end-systolic volume; LVEF=left ventricular ejection fraction; GLS 4ChS=global longitudinal strain measured at four-chamber ultrasonic view obtained from speckle-tracking echocardiography; GLS 2ChS=global longitudinal strain measured at two-chamber ultrasonic view obtained from speckle-tracking echocardiography; GLS 3ChS=global longitudinal strain measured at three-chamber ultrasonic view obtained from speckle-tracking echocardiography; SAXBC=circumferential strain measured at the basal ventricular level, ultrasonic view; SAXAC=circumferential strain measured at the apical ventricular level, ultrasonic view; SAXMC=circumferential strain measured at the mid-ventricular level, ultrasonic view
Analyses were carried out for comparing antecedent Kawasaki patients and controls and also patients without coronary aneurysms and controls.
aLevy et alReference Levy, Machefsky and Sanchez 13
* p⩽0.05 level for antecedent Kawasaki patients and controls
** The statistical difference was not significant (p>0.05) for coronary aneurysm (−) versus coronary aneurysm (+) in the Kawasaki disease group
*** The statistical difference was significant for coronary aneurysm (−) in antecedent Kawasaki patients versus controls
Left ventricular strain analysis of patients with antecedent Kawasaki disease and controls using speckle-tracking echocardiography
The independent samples t-tests for left ventricular deformation values using speckle-tracking echocardiography were significantly lower in patients with antecedent Kawasaki disease compared with controls at the basal inferoseptal, basal anterolateral, apical septal, and apical inferior segments of the left ventricle (p⩽0.05) (Table 5).Reference Levy, Machefsky and Sanchez 13
Left ventricular deformation values of patients without coronary aneurysms were also significantly lower than controls at the basal inferoseptal, apical septal, and apical inferior segments of the left ventricle (p⩽0.05) (Table 5).
To clarify the laboratory and clinical correlates of the lower left ventricular deformation indices measured during the study, we compared controls with patients with antecedent Kawasaki disease regarding receiving intravenous immunoglobulin therapy before and after 10 days of fever, age at diagnosis (older/younger than 20 months), platelet count, haemoglobin level, alanine transaminase level, and presence or absence of pyuria during the course of the disease. There was no statistically significant difference in deformation values of left ventricular segments in patients with antecedent Kawasaki disease according to all these markers except pyuria. The deformation values of these patients without pyuria during the course of the disease were significantly higher at the mid anterior, mid anteroseptal, apical anterior, and apical inferior segments of the left ventricle (p<0.05) compared with those of patients with pyuria. In addition, the global longitudinal strain – measured in the four-chamber view – values were statistically higher in patients with antecedent Kawasaki disease without pyuria than in those with antecedent Kawasaki disease with pyuria (Table 6).
Table 6 Current strain measurements of left ventricular segments with speckle-trackıng echocardıography between patients with antecedent Kawasaki disease having pyuria and having no pyuria during the acute phase of the disease

AHA=American Heart Association; GLS 4ChS, four-chamber view longitudinal strain (%); SAXAC=circumferential strain at apical level (%), speckle-tracking echocardiography
* Statistically significant at 0.05 difference level
Discussion
Using speckle-tracking echocardiography during mid-term follow-up, we have shown that the left ventricular systolic strain was impaired in the basal and apical segments of patients with antecedent Kawasaki disease. Among antecedent Kawasaki disease patients, however, we could not find any difference in myocardial deformation values of the left ventricle between those with and without coronary aneurysms.
Speckle-tracking echocardiography results in patients
In the present study, left ventricular strain was impaired in the basal and apical segments of patients with antecedent Kawasaki disease. It has been shown that changes in myocardial deformation can be detected in young patients with muscular dystrophy, which decreases with ageReference Mertens, Ganame and Claus 14 , Reference Marcus, Barends and Morava-Kozicz 15 , in mitochondrial diseases, and in Friedreich’s ataxiaReference Dedobbeleer, Rai and Donal 16 , whereas the ejection fraction remains in the normal range. Childhood obesity and diabetes mellitus in children has been found to be associated with reduced longitudinal left ventricular strain and global strain.Reference Nakai, Takeuchi and Nishikage 17 StudiesReference Poterucha, Kutty and Lindquist 18 – Reference Fallah-Rad, Walker and Wassef 20 on chemotherapy survivors and cardiac transplantation patients undergoing regular echo surveillance for which early detection of myocardial dysfunction is of utmost importance showed significant changes in longitudinal strain 4–6 months after starting chemotherapy, whereas significant changes in ejection fraction could only be detected at eight months. All these findings strongly indicate that abnormal strain measurements reflect early changes in myocardial function before a decline in ejection fraction. Thus, we attempted to demonstrate whether this subclinical myocardial compromise detected by segmental strain could be attributed to a particular region affected by antecedent Kawasaki Disease. Could any segment be an index point of compromise or a long-lasting effect of coronary artery involvement during the acute course of the disease, and therefore an explanation for the low cardiac strain?
In a comparison between antecedent Kawasaki patients and healthy controls, we found that basal inferoseptal, basal anterolateral, apical septal, and apical inferior segments had significantly lower myocardial strains than controls. Low values of deformation measurements at the basal and apical segments in our patients could have possibly indicated that cardiac dysfunction resulted because of coronary lesions. There was no significant difference between the left ventricular segments of patients with or without coronary lesions, although this could be attributed to the small number of antecedent Kawasaki disease patients with coronary aneurysm.
Possible mechanisms of low cardiac strain in antecedent Kawasaki patients
Finding no correlation between coronary artery involvement and low myocardial segmental strain indicates that there must be other contributing factors other than myocardial ischaemia caused by coronary involvement. Xu et alReference Xu, Ding and Lv 9 reported that speckle-tracking imaging of two-dimensional left ventricular systolic strain is accurate in evaluating left ventricular function during different phases of Kawasaki disease, and concluded that decreased global longitudinal strain should be regarded as a myocardial functional disturbance associated with Kawasaki myocarditis. In another study by Yu et al,Reference Yu, Wong and Cheung 21 of adolescents and young adults with a history of Kawasaki disease, impairment of left ventricular mechanics was found to occur and be worse in patients with, compared to those without, coronary complications. Myocardial biopsy studiesReference Yutani, Go and Kamiya 22 , Reference Fujiwara and Hamashima 23 of patients without coronary aneurysms and biochemical markers reflecting the inflammatory process of myocardial response during the acute phase indicate myocarditis.Reference Dahdah, Siles and Fournier 24 Although there are reports stating that left ventricular strain impairment during the acute phase of Kawasaki diseaseReference Yu, Choi and Kim 8 , Reference Newburger, Sanders and Burns 25 and the inflammatory involvement of the myocardium recovered after its acute phaseReference Xu, Ding and Lv 9 , the present study is the first to observe regional systolic strain impairment during mid-term follow-up of patients with antecedent Kawasaki disease, irrespective of coronary involvement.
Many clinicians, including us, who treat patients with Kawasaki disease, appear to be more interested in the direct effects of Kawasaki disease vasculitis on the coronary arteries than in the possibility of early onset of adult disease. As DahdahReference Dahdah 26 stated, myocarditis is the hidden face of the moon in Kawasaki disease. If the inflammatory involvement of the myocardium results in regional myocardial functional impairment 4 years after the onset of the disease, this means its long-term consequences deserve a serious look and a methodological follow-up.
We observed high C-reactive protein levels during the acute phase of Kawasaki disease to be associated with coronary artery aneurysms – results that are in agreement with previous studies.Reference Xu, Ding and Lv 9 – Reference Levy, Machefsky and Sanchez 13 In our study, patients with pyuria in the acute phase of the disease had lower deformation values than did those without pyuria. Pyuria has been variably reportedReference Watanabe, Abe and Sato 27 , Reference Toru 28 in 33%–62% of patients with acute Kawasaki disease, and it has been recognised in the recent American Heart Association guidelines as a laboratory finding supportive of the diagnosis of Kawasaki disease.Reference Newburger, Takahashi and Gerber 10 MasonReference Mason, Schneider and Takahashi 29 suggested that coronary artery aneurysms are associated with sterile pyuria in Kawasaki disease; however, SephiaReference Sepahi, Miri and Ahmadi 30 reported that pyuria could not be considered as a predicting factor for coronary artery aneurysms. Kawasaki disease patients with pyuria exhibit severe inflammatory reactions,Reference Toru 28 as well as severe myocarditis during the acute phase, which may explain the impaired strain values in patients with antecedent Kawasaki disease.
Follow-up in patients with antecedent Kawasaki disease
Senzaki et alReference Senzaki 31 discussed the ongoing controversy regarding an appropriate follow-up protocol for patients without any evidence of coronary artery lesions. The guidelines 32 mostly refer to the status of coronary artery involvement. The optimal frequency and intensity of follow-up for patients without coronary artery aneurysms are unknown.Reference Watanabe, Abe and Sato 27 In the review by Gordon et al,Reference Gordon, Kahn and Burns 3 the lack of reports about the longitudinal outcome in Kawasaki patients who were treated with intravenous immunoglobulin during the acute illness phase was emphasised, and they stated that the fate of intravenous immunoglobulin-treated children who had no lesions detected by echocardiography as a result of the acute vasculitis is unknown. It is obvious that the lower myocardial deformation observed even in patients without coronary aneurysms indicates that intravenous immunoglobulin used to control the disease is insufficient to prevent possible devastating cardiac effects. It follows, therefore, that preventive therapeutic medications/interventions should be taken into account for the long-term follow-up of patients with antecedent Kawasaki disease.
Limitations of the present study include the small number of patients at a single centre with coronary aneurysms, limiting statistical analysis and the extent of our conclusions. Given the few patients with coronary aneurysm and the relatively short follow-up time for Kawasaki disease, we were unable to compare the results from the onset of disease for an accurate estimation of longitudinal myocardial compromise in different groups with speckle-tracking echocardiography and compare it with conventional echocardiographic methods over time. We have only one data set of strain values, although the results show a differece between patients and controls. Our conclusions would have been strengthened if we had data from consecutive longitudinal measurements. We did not perform reproducibility tests.
This study has shown that left ventricular strain in antecedent Kawasaki disease is impaired in basal and apical segments. Using speckle-tracking echocardiography during mid-term follow-up, however, we found no association between left ventricular dysfunction and coronary dilatation. The strain value in our patients with antecedent Kawasaki disease was lower than controls, and these values, which were still within normal ranges, may be an early predictor of impaired functional outcome in the longer term. In a meta-analysis by Colquitt et al, a one standard deviation change in global longitudinal strain of the left ventricle was observed to be a stronger predictor of all-cause mortality, when compared with the same change in left ventricular ejection fraction in both univariable and multivariable models.Reference Colquitt and Pignatelli 33 The prognostic value of this information seems likely to be superior to that provided by left ventricular ejection fraction during follow-up, and will require further longitudinal follow-up data with larger patient series. We think it important to regularly measure the left ventricular function of patients with antecedent Kawasaki disease irrespective of coronary artery involvement. In the coming years, patients with antecedent Kawasaki disease may differ from healthy individuals regarding traditional cardiovascular risk factors, which will accumulate as this population ages. Cardiac regional strain measurements during follow-up could help differentiate the effects of antecedent Kawasaki disease from the effects of these risk factors. This may potentially result in the early diagnosis and treatment of antecedent, high-risk, Kawasaki patients, which would have clear benefits for their future health. Speckle-tracking imaging is a simple, non-invasive technique, which we think could replace conventional echocardiography when performing this vital assessment of cardiac function. Further multicentre, larger, longitudinal follow-up, cardiac strain studies are needed to better understand the maturational patterns of patients with antecedent Kawasaki disease and their longer-term outcomes.
Acknowledgements
The authors would like to thank Ms. Ummu Kımmet from Philips imaging systems for her assistance in technical support. Authors’ Contributions: All authors were involved in drafting the article or revising it critically for important intellectual content. All authors approved the final version to be submitted for publication. Dr Dedeoglu had full access to all the data and was responsible for the accuracy of the data analysis. Study design: Kasapçopur Ö, Dedeoglu R, and Öztunç F. Acquisition of data: Dr Şahin, Dr Atik, Dr Barut, and Dr Adroviç. Analysis and interpretation of data: Kasapçopur Ö, Dedeoglu R, and Cengiz D.
Financial Support
No funding was received from any public or special commercial sectors for this manuscript.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this study comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Istanbul University Cerrapasa medical faculty ethics committe.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951117000580










