Recent surveillance studies from Africa, Asia, Australasia, and Oceania have documented the high prevalence of latent rheumatic heart disease in schoolchildren, which is defined as asymptomatic rheumatic heart disease detected on echocardiography in the absence of a history of preceding acute rheumatic fever.Reference Rothenbuhler, O’Sullivan and Stortecky 1 These studies have also demonstrated the superiority of portable echocardiography over auscultation with the ordinary stethoscope in detecting the early structural and functional changes of rheumatic heart disease.Reference Saxena, Ramakrishnan and Roy 2 – Reference Grimaldi, Ammirati, Mirabel and Marijon 13 The recently published revised Jones criteria also refer to the importance of asymptomatic changes seen on echocardiography.Reference Gewitz, Baltimore and Tani 14 The World Heart Federation has developed evidence-based criteria for the echocardiographic diagnosis of rheumatic heart disease that serve as the new standard for research in this field;Reference Remenyi, Wilson and Steer 15 however, portable echocardiography machines are expensive, and the screening protocols are complex, requiring highly trained healthcare professionals for acquisition and interpretation of the images.Reference Mirabel, Celermajer and Ferreira 16 Furthermore, the scanning protocol requires the acquisition of multiple images over a 10- to 20-minute period, which limits the number of patients that can be screened in field conditions. Therefore, there is a need to develop simple, affordable, and reliable screening modalities and protocols for latent rheumatic heart disease for epidemiological studies.Reference Zühlke and Mayosi 17
Computer-assisted auscultation, a promising modality in screening for structural heart disease, uses a digital stethoscope combined with acoustic neural networking to provide a visual display of heart sounds and murmurs, and also analyses the recordings to distinguish between innocent and pathological murmurs.Reference Zühlke, Myer and Mayosi 18 Therefore, auscultation using a digital stethoscope together with an objective computer algorithm to identify pathological murmurs may improve the sensitivity and positive predictive value of cardiac auscultation. The performance of computer-assisted auscultation in the detection of pathological murmur in the context of latent rheumatic heart disease is not known.
Hand-held echocardiography machines represent an important advancement over standard portable ultrasound equipment due to their small size and lower cost, but the lack of Doppler capabilities hampers their widespread use. Nevertheless, recent reports have found that, compared with the use of portable echocardiography and full World Heart Federation criteria, hand-held portable echocardiography is sensitive and specific for the detection of latent rheumatic heart disease using modified World Heart Federation criteria,Reference Beaton, Aliku and Okello 19 This protocol, however, requires the acquisition of multiple images and expertise in recognising morphological features of rheumatic heart disease. We have, therefore, designed a simple protocol called FOCUS – that is, A FOCussed method Utilising hand-held echocardiography in Screening for rheumatic heart disease – which aims to identify one cardiac abnormality in the shortest possible time by a minimally trained observer for the diagnosis of rheumatic heart disease. We hypothesised that a simple protocol, using the single mitral regurgitation jet-length criterion of Mirabel et alReference Mirabel, Celermajer and Ferreira 20 with a hand-held echocardiogram, may have high sensitivity and specificity in detecting rheumatic heart disease in asymptomatic schoolchildren.
The aim of this study was to assess the diagnostic utility – sensitivity, specificity, negative and positive predictive values, test efficiency, and time – of computer-assisted cardiac auscultation and the focussed protocol using hand-held echocardiography in the diagnosis of latent rheumatic heart disease in a population with a high burden of asymptomatic rheumatic heart disease. Computer-assisted auscultation was also compared with standard auscultation by a cardiologist for the detection of pathological murmur.
Materials and methods
Study population
The original echocardiographic screening study of latent rheumatic heart disease was conducted on a random sample of 2720 scholars from the Vanguard communities of Cape Town, which are made up of the two suburbs of Bonteheuwel (n=1303) and Langa (n=1417).Reference Engel, Haileamlak and Zühlke 21 Following the previous rheumatic heart disease screening at this site, a nested case–control study commenced in August, 2013 and continued until September, 2014. Cases were scholars previously diagnosed with asymptomatic rheumatic heart disease on screening echocardiography with persistent disease. Cases were either classified as definite or borderline cases of rheumatic heart disease in those younger than 20 years of age, whereas cases were categorised as definite in those older than 20 years of age according to the World Health Federation criteria. The controls were normal healthy scholars previously enrolled in the screening study who were matched for age, school grade, and residential area. Cases and the controls were invited to a research clinic at Groote Schuur Hospital in Cape Town. The single reviewing physician (L.J.Z.) was blinded to their echocardiographic status found during the original screening study. This study was approved by the Human Research Ethics Committee of the University of Cape Town and the Departments of Health and Education of the Western Cape Government.Reference Engel, Zühlke and Robertson 22
Test methods
Recording heart sounds using computer-assisted auscultation and the Zargis cardioscan system
Patients were examined in a quiet room. They were first examined for the presence of pathological murmur using a standard stethoscope, and then subjected to computer-assisted auscultation. When using the Zargis® system (Zargis Medical, Princeton, New Jersey, United States of America), auscultation was performed using a Bluetooth Littman® electronic stethoscope Littman Bluetooth, Minnesota, United States of America. The heart sounds and murmurs were transmitted to a computer loaded with Cardioscan® software Zargis Cardioscan, Princeton, New Jersey, United States of America. The heart sounds were recorded at four positions for 20 seconds at each site – that is, mitral, tricuspid, pulmonary, and aortic areas. The software analysed the recordings for up to 1 minute and then displayed findings as abnormal or normal (Fig 1). Data arising from the computer-assisted auscultation were stored in a password-controlled netbook in zac files for the Zargis® system. These files were downloaded to a portable hard-drive for archiving and analysis.
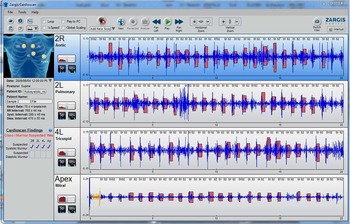
Figure 1 Zargis user interface in the case of an abnormal murmur being detected.
Focussed echocardiography protocol utilising hand-held echocardiography
The cases and healthy controls underwent the focussed echocardiography protocol on the General Electric V-Scan® ultra-portable hand-held machine (General Eletric Company, Fairfield, CT, United States of America), followed by a full echocardiogram using a standard portable machine (Philips CX 50, Philips Healthcare, DA Best, The Netherlands). A single operator (L.J.Z.) performed all the echocardiographic assessments. The focussed echocardiography protocol comprised an interrogation of the mitral valve in the long-axis parasternal view, first without and then with color Doppler. Any regurgitation was recorded, and the mitral regurgitation jet-length was measured from the vena contracta to the last pixel of the regurgitant color Doppler map using the V-Scan radius measuring tool. A measurement where the mitral regurgitation jet-length was ⩾2 cm constituted a positive result (Fig 2). The echocardiograms were scored according to the focussed protocol as rheumatic heart disease screen-positive or screen-negative. All the patients also underwent a comprehensive echocardiogram on a portable echocardiography machine and were categorised using the World Heart Federation criteria to confirm definite, borderline rheumatic heart disease in those younger than 20, or a normal finding.Reference Remenyi, Wilson and Steer 23
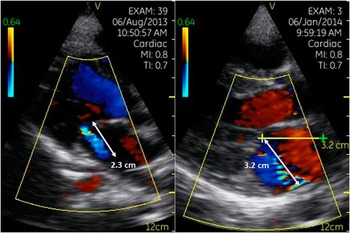
Figure 2 Screen-positive mitral regurgitation jets.
Statistical methods
The clinical characteristics of the patients were described as the mean and standard deviation for normally distributed data and as the median and interquartile range for skewed data. A sample size of 75 patients would determine the sensitivity, with 95% confidence and a precision of >1%. A minimum sensitivity of >90% is regarded as a characteristic of an acceptable screening test.Reference Akobeng 24 – Reference Zhou, Obuchowski and McClish 26 We also computed the diagnostic odds ratio, which is a single measure of effectiveness of a diagnostic test that is independent of prevalence.Reference Glas, Lijmer, Prins, Bonsel and Bossuyt 25 A diagnostic odds ratio >1 is indicative of a discriminatory test, whereas a diagnostic odds ratio that is >400 indicates an acceptable effect size.
The findings of the focussed protocol were recorded as normal or “screen-positive” for rheumatic heart disease, whereas the computer-assisted auscultation results were scored as either normal or abnormal. The sensitivity and specificity of both tests to detect patients with definite and borderline rheumatic heart disease, together and separately, are reported with the echocardiographic diagnosis of rheumatic heart disease as the standard reference, utilising the full World Heart Federation criteria on images acquired by means of a standard portable echocardiography machine. Sensitivity and specificity analyses were carried out using contingency tables, 95% confidence intervals were calculated using the “cii” command in STATA, and positive and negative predictive values for the alternative screening modalities were determined, along with the reliability, or percentage correct, of the test. In the cases where the sensitivity or the specificity equalled zero, the contingency tables were all adjusted by adding 0.5, according to the method described by Glas et al,Reference Glas, Lijmer, Prins, Bonsel and Bossuyt 25 to report the diagnostic odds ratio.
All the statistical tests were two-sided at α=0.05. Data were captured into an Epi Info® database (CDC, Atlanta, Georgia, United States of America) and analysed using STATA® version 12 (StataCorp LP, Station Road, Texas, United States of America). The standards for reporting of diagnostic accuracy studies (STARD) and strengthening the reporting of observational studies in epidemiology (STROBE) criteria were used for the analysis and reporting of this study.Reference Bossuyt, Reitsma and Bruns 27 , Reference Vandenbroucke, von Elm and Altman 28
Results
Characteristics of patients
There were 27 scholars with either definite or borderline rheumatic heart disease, according to the World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease, recruited between August, 2013 and September, 2014. The median age was 17 – interquartile range from 14 to 20 – their minimum age was 8 years, their maximum age was 24 years, and 59.2% were female. There were 66 healthy controls with a median age of 17 years – interquartile range from 13 to 21 – minimum age of 10 years, and maximum age of 25 years; 73.1% were female.
Performance of standard and computer-assisted auscultation and hand-held and portable echocardiography
Among all, six patients had clinically detected murmurs, five of whom were cases of latent rheumatic heart disease with a pathological murmur (5/27, 18.5%), and a single control with an innocent murmur (1/66, 1.5%). Computer-assisted auscultation detected one scholar with an abnormal murmur, diagnosed as latent rheumatic heart disease (1/27, 3.7%); 21 scholars (21/27, 77.8%) were identified as screen-positive using the focussed hand-held echocardiography protocol.
Computer-assisted auscultation: test characteristics
The sensitivity of the automated auscultation decision tool to detect an abnormal murmur in cases of definite or borderline rheumatic heart disease was 4% (95% CI 1.0–20.4%). The specificity was 93.7% (95% CI 84.5–98.3%). The positive predictive value was 20% (95% CI 5–71.6%), whereas the negative predictive value was 71.1% (95% CI 60.1–80.5%). The likelihood ratios were 0.02 (positive likelihood ratio) and 0.41 (negative likelihood ratio), respectively, with a percentage of abnormal murmurs in cases of rheumatic heart disease equalling 68.2% and a diagnostic odds of 0.04 as shown in Table 1.
Table 1 Test characteristics for detecting definite and borderline RHD.

CI=confidence intervals; FOCUS=A FOCussed method Utilising hand-held echocardiography in Screening for RHD; LR+=positive likelihood ratio test; LR−=negative likelihood ratio test; NPV=negative predictive value; PPV=positive predictive value; RHD=rheumatic heart disease
* Contingency tables adjusted for 0 values according to Glas et alReference Glas, Lijmer, Prins, Bonsel and Bossuyt 25
In reviewing the two categories of echocardiographic rheumatic heart disease separately, the sensitivity for detecting definite and borderline disease was 9.1% (95% CI 0.2–41.3%) and 0%, respectively. The specificity for detecting abnormal murmurs in both definite and borderline disease was 95.2% (95% CI 86.5–98.9%). The positive predictive value was 20% (95% CI 5–71.6%) for definite disease. The negative predictive values were 85.5% (95% CI 74.9–92.8%) for definite and 80.8% (95% CI 69.9–89.1%) for borderline disease, respectively. The test reliability was 81.1% for definite disease and 76.6% for borderline disease, with a diagnostic odds ratio of 1.48 and 0.46 (adjusted) for detecting definite and borderline disease, respectively (Tables 2 and 3).
Table 2 Performance characteristics of computer-assisted auscultation and focussed hand-held echocardiography to identify definite RHD.

CI=confidence intervals; FOCUS=A FOCussed method Utilising hand-held echocardiography in Screening for RHD; LR+=positive likelihood ratio test; LR−=negative likelihood ratio test; NPV=negative predictive value; PPV=positive predictive value; RHD=rheumatic heart disease
* Contingency tables adjusted for 0 values according to Glas et alReference Glas, Lijmer, Prins, Bonsel and Bossuyt 25
Table 3 Performance characteristics of alternate modalities in identifying borderline subclinical RHD.

CI=confidence intervals; FOCUS=A FOCussed method Utilising hand-held echocardiography in Screening for RHD; LR+=positive likelihood ratio test; LR−=negative likelihood ratio test; NPV=negative predictive value; PPV=positive predictive value; RHD=rheumatic heart disease
* Contingency tables adjusted for 0 values according to Glas et alReference Glas, Lijmer, Prins, Bonsel and Bossuyt 25
FOCUS protocol: test characteristics
The average time to record the images using the focussed protocol with hand-held echocardiography was just under 2 minutes (mean 117±22 seconds). No technical difficulties were encountered. The sensitivity of the FOCUS protocol together with hand-held echocardiography in order to identify correctly the cases of definite or borderline rheumatic heart disease was 80.8% (95% CI 60.6–93.4%). The specificity was 100%. Accordingly, the positive predictive value was 100%, whereas the negative predictive value was 92.5% (95% CI 83.4–97.5%). The likelihood ratios were 43 (positive likelihood ratio) and 0.08 (negative likelihood ratio), respectively, with a percentage of correct diagnosis of 94.3% and an adjusted diagnostic odds of 489.
Reviewing the two categories of borderline and definite rheumatic heart disease individually shows that the test statistics improved for definite disease and worsened for borderline disease. The sensitivity for definite and borderline disease was 92.3% (95% CI 63.9–99.8%) and 69.2% (95% CI 38.5–90.9%), respectively. The specificity for detecting both definite and borderline disease was 100%. The test reliability was 98.7% for detecting definite disease and 94.7% for detecting borderline disease, with the adjusted diagnostic odds ratio being 1041 and 263.9 for detecting definite and borderline disease, respectively (Tables 2 and 3).
Discussion
Our study has three key findings. First, computer-assisted auscultation performed dismally in detecting latent rheumatic heart disease. The modality was worse than standard auscultation in detecting cases of pathological murmur associated with latent rheumatic heart disease (Fig 3). Second, focussed hand-held echocardiography, which can be carried out within 2 minutes/study, has a moderate sensitivity and high specificity and diagnostic odds for detecting latent rheumatic heart disease. Finally, the sensitivity of the focused hand-held echocardiography protocol is higher for definite rheumatic heart disease compared with borderline cases.
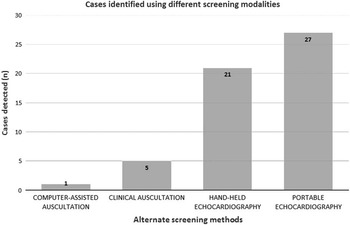
Figure 3 Scholars identified using alternative screening methods.
The focussed protocol defines a simplified method of using a single criterion with hand-held echocardiography to screen for asymptomatic rheumatic heart disease. This confirms the need as demonstrated by Reeves,Reference Reeves, Kado and Brook 29 Mirabel,Reference Mirabel, Celermajer and Ferreira 20 and BeatonReference Beaton, Okello, Lwabi, Mondo, McCarter and Sable 7 , Reference Beaton, Aliku and Okello 19 , Reference Beaton, Lu and Aliku 30 to develop a simple method for the large-scale screening of rheumatic heart disease in low-resource settings. Reeves and Mirabel investigated the use of shorter echocardiography protocols with standard portable echocardiography machines, whereas Beaton used the hand-held echocardiography machine with modified World Heart Federation criteria. Mirabel also recently reported the use of a short echocardiography protocol with hand-held echocardiography, utilising mitral and aortic regurgitant jet lengths.Reference Mirabel, Bacquelin and Tafflet 31 Lu et alReference Lu, Sable and Ensing 32 found that mitral regurgitation >1.5 cm and any aortic incompetence was superior to mitral regurgitation alone in optimising sensitivity and specificity. Our study used only the single-jet criterion with hand-held echocardiography for the screening of latent rheumatic heart disease. This single criterion was chosen because pathological mitral regurgitation is an independent predictor of the progression or persistence of mild valvular lesions in latent rheumatic heart disease (Zühlke, unpublished data).
Thus far, abbreviated studies using hand-held echocardiography have predominantly screened younger schoolchildren.Reference Beaton, Lu and Aliku 30 – Reference Godown, Lu and Beaton 34 Our findings indicate that hand-held echocardiography can be utilised in an older age group. This needs further validation but may be of importance in planning future echo screening programmes for vulnerable populations such as pregnant women.Reference Otto, Saether, Banteyrga, Haugen and Skjaerpe 35
Screening using a test with high sensitivity but low specificity will result in many patients needing expensive further investigation, which is an untenable situation in low-resource settings. This was the criticism for the screening criteria used before the publication of the World Heart Federation criteria.Reference Kothari 36 , Reference DeGroff 37 We have found, however, that this protocol has sufficiently high sensitivity and reliability to be feasible for use as a screening test for definite rheumatic heart disease in high-prevalence communities. It has already been established that the World Heart Federation criteria are appropriately specific for their confirmatory function.Reference Roberts, Maguire and Brown 38
We acknowledge that the focussed echocardiography protocol criteria failed to detect a proportion of cases of borderline disease, specifically ones that were only affecting the morphology of the mitral or aortic valves (Table 4). To date, screening studies have been characterised by findings of mitral regurgitation in over 95% of definite disease.Reference Paar, Berrios and Rose 9 , Reference Reeves, Kado and Brook 29 These have also been found to be the most useful in pilot studies and other short protocols.Reference Beaton, Aliku and Okello 19 , Reference Reeves, Kado and Brook 29 , Reference Colquhoun, Carapetis and Kado 39 Although the role of morphological abnormalities is clear in the determination of definite disease, borderline disease has been shown to have a variable outcome, from worsening to normalising in a significant proportion. The implications of missing a large number of borderline cases are unknown.
Table 4 Classification of definite and borderline cases.

AR=aortic regurgitation; AV=aortic valve; CAA=computer-assisted auscultation; FOCUS=A FOCussed method Utilising hand-held echocardiography in Screening for RHD; MR=mitral regurgitation; MV=mitral valve; RHD=rheumatic heart disease
Several reports demonstrate that cardiac auscultation is insufficiently sensitive when screening for rheumatic heart disease.Reference Marijon, Ou and Celermajer 3 , Reference Bhaya, Panwar, Beniwal and Panwar 10 , Reference Godown, Lu and Beaton 34 , Reference Saxena, Zühlke and Wilson 40 – Reference Kane, Mirabel and Toure 42 The use of an automated decision in combination with digital auscultation may improve the sensitivity and specificity of murmur detection,Reference Steer, Kado and Wilson 43 , Reference Tavel 44 and the method, furthermore, has merit in terms of the teaching and training of healthcare professionals.Reference Tavel 45 We have demonstrated unequivocally, however, that computer-assisted auscultation has no place in the screening for asymptomatic rheumatic heart disease, faring worse than ordinary cardiac auscultation. Although standard cardiac auscultation remains cheap, easy to use in all settings, and shows increased specificity when used with an algorithm, its role as a screening tool for large-scale rheumatic heart disease is limited.
The World Health Organisation has identified the establishment of national programmes for the prevention and control of acute rheumatic fever/rheumatic heart disease as a national priority for high-prevalence communities.Reference Zühlke, Myer and Mayosi 46 Thus far, however, the vast majority of screening programmes have been isolated research projects, not embedded within existing control programmes. This protocol has the potential to position future screening programmes within rheumatic heart disease prevention and control programmes. In addition, the point-of-care application of hand-held echocardiography to diagnose systolic dysfunction, advance antenatal care, and include vascular scanning aligns this protocol with integrated models of care, where rheumatic heart disease could form one of the diseases easily screened for and managed by primary-care teams; 47 however, further studies are needed to show that screening changes outcomes, before it is likely to be adopted for wide use.
The costs reported by Reeves et al,Reference Reeves, Kado and Brook 29 using a non-cardiologist reviewer, a lower-cost echocardiography machine, and a shortened scanning time, were remarkably low at a cost-per-patient screened of US$2.07 and a cost-per-case of definite rheumatic heart disease detected of US$37.75. We envision that this protocol will be similarly cost-efficient, and thus affordable, in low-income countries. An additional cost of screening relates to the expert personnel needed to perform and review echocardiograms. With this method, it is likely to be relatively easy to train non-expert operators to perform scans, thus reducing costs considerably. A recent report has suggested that screening for latent rheumatic heart disease is cost-effective.Reference Mayosi 48 Further research on the cost-effectiveness of a programme based on this protocol and the training and implementation of a non-cardiologist is the natural extension of this study. A study is underway to train radiology staff to implement this protocol and screen larger numbers of children in Zambia.Reference Zachariah and Samnaliev 49
This study has several limitations. We used a small sample of cases of asymptomatic rheumatic heart disease; however, the confidence intervals around the estimates were small, suggesting that the sample size was adequate for the purposes of this study. Second, the study was conducted by a cardiologist who performed the auscultation and the echocardiography. The findings may, therefore, not be generalisable to the performance of this study by minimally trained health staff. Third, we did not evaluate the utility of a shorter mitral regurgitant jets <2 cm as performed by Lu et alReference Lu, Sable and Ensing 32 We elected to use the standard definition of 2 cm or greater as pathological.Reference Mayosi, Gamra, Dangou and Kasonde 50 Finally, the use of a single observer means that the reproducibility of the measurements of this study is unknown.
Conclusion
FOCUS, a FOCused method Utilising hand-held echocardiography in Screening for rheumatic heart disease, is a simple, brief, sensitive, and highly specific method of screening for rheumatic heart disease that may be suited for low-income settings, as it utilises hand-held ultra-portable echocardiography and simple diagnostic criteria; however, computer-assisted auscultation is not a suitable screening modality for latent rheumatic heart disease due to extremely low sensitivity.
Acknowledgements
The authors are grateful to Peggy Mgwayi and Veronica Francis for their assistance with this project.
Financial Support
This study was supported in part by research grants from the Life Healthcare Foundation, the South African Medical Research Council, the Lily and Ernst Hausmann Research Trust, the Else Kröner Fresenius Foundation, the University of Cape Town, the National Research Foundation of South Africa, the World Heart Federation, and the Novartis Biomedical Research Institute. Dr Liesl Zühlke was funded by the Discovery Foundation, the US National Institutes of Health Fogarty International Clinical Research Fellowship, Thrasher Research Fund Early Career Award, Wellcome Trust Clinical Infectious Disease Research Initiative (CIDRI) Research Officer Award, and the Hamilton Naki Clinical Scholarship.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all the procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation of South Africa and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committee, the Human Research Ethics Committee, University of Cape Town. Written informed consent was obtained from the parents of all the patients, in addition to written assent by patients older than 8 years.










