Neonates, infants, and children with CHD have been shown to have a heightened risk of central venous catheter-related thrombotic events.Reference Giglia, Massicotte and Tweddell 1 – Reference Murphy and Mastropietro 6 Prophylactic enoxaparin may be used to prevent central venous catheter-associated thrombus formation in these patients, but few data exist to guide practitioners on optimal dosing strategies in young children.Reference Giglia, Massicotte and Tweddell 1 , Reference Monagle and Karl 4 – Reference Hanson, Punzalan and Arca 9
Enoxaparin therapy is monitored using anti-factor Xa assays in children. In the absence of renal impairment or other conditions, such as obesity or a hypermetabolic state, anti-factor Xa concentrations are not routinely monitored in adults. Infants and young children, however, require relatively higher enoxaparin doses per kilogram of body weight than adults to achieve similar anti-factor Xa concentrations. This variation is probably because of differences in volume of distribution, elimination half-life, and renal function across the span of childhood development. Furthermore, the undeveloped haemostatic system in neonates and infants may also affect enoxaparin dosing requirements.Reference Andrew, Paes and Milner 10 A small number of studies support the need for higher therapeutic doses of enoxaparin in critically ill children,Reference Diab, Ramakrishnan and Ferrell 7 , Reference Schloemer, Abu-Sultaneh and Hanson 11 , Reference Sanchez de Toledo, Gunawardena and Munoz 12 although little data exist on the effectiveness of current prophylactic dosing regimens in the paediatric population – for example, children with CHD.
We aimed to determine whether standard prophylactic enoxaparin dosing regimens are effective in children with CHD at achieving anti-factor Xa concentrations within the goal range of 0.25–0.49 IU/ml for thrombus prophylaxis.
Materials and methods
A central venous catheter-related thrombus prophylaxis protocol for children with CHD was implemented at our institution in January, 2016. This protocol is shown in Figure 1. Standard prophylactic enoxaparin dosing regimens – for example, 0.75 mg/kg/dose subcutaneously every 12 hours for patients <2 months of age and 0.5 mg/kg/dose subcutaneously every 12 hours for patients ≥2 months of age – were used for all empiric prophylaxis.Reference Monagle, Chan and Goldenberg 5 Doses were titrated by a clinical pharmacist to a target anti-factor Xa range of 0.25–0.49 IU/ml. This goal range was chosen based on local experience in combination with literature demonstrating the lack of efficacy when targeting a lower prophylactic range (0.1–0.3 IU/ml) in children with central venous catheters receiving thromboprophylaxis.Reference Massicotte, Julian and Gent 13 , Reference Schroeder, Axelrod and Silverman 14 Subcutaneous catheters, for example Insuflon®, were not used in any patients receiving prophylactic enoxaparin therapy. Before implementation of this protocol, anti-factor Xa levels were not routinely monitored in patients receiving prophylactic enoxaparin at our institution.
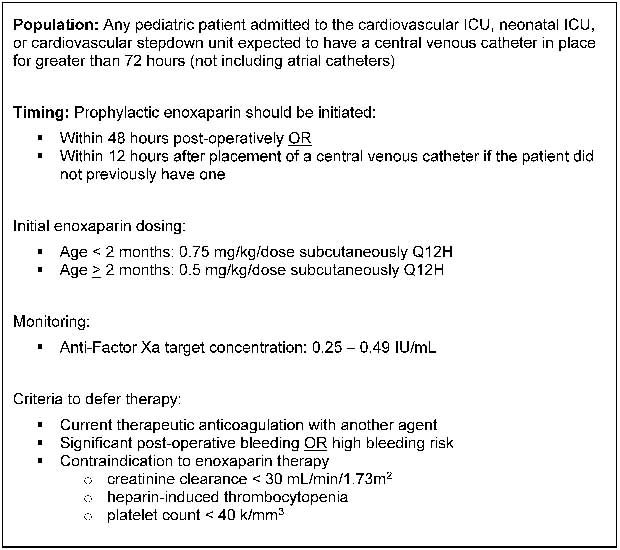
Figure 1 Central venous catheter thrombosis prophylaxis protocol.
This retrospective review, which was approved by the Indiana University Institutional Review Board, assessed dosing requirements for children treated with prophylactic enoxaparin according to this protocol (Fig 1) at Riley Hospital for Children between January 25, 2016 and August 31, 2016. Eligible patients were <2 years of age, had a diagnosis of CHD, and documentation of at least one appropriately drawn (4–6 hours post-enoxaparin dose) steady-state, drawn beyond at least the third enoxaparin dose after initiation or after any dosing changes, anti-factor Xa concentration. Exclusion criteria included significant renal dysfunction during enoxaparin treatment, defined as any form of renal replacement therapy or creatinine clearance <30 ml/min/1.73 m2. Patients were also excluded if they had a confirmed diagnosis of heparin-induced thrombocytopaenia, platelet count <40,000/mm3, or concurrent therapeutic anti-coagulation. Eligible patients were identified via a retrospective query of all patients who received enoxaparin during the study period. Data relating to patient demographics, renal function, enoxaparin dosing, and anti-factor Xa concentrations were collected from the electronic medical record.
Descriptive statistics were used to characterise the study population, enoxaparin dosing, and anti-factor Xa concentrations. Data are provided as median (25, 75%) or absolute counts (%) as appropriate. Creatinine clearance was estimated using the bedside Schwartz equation.Reference Schwartz, Munoz and Schneider 15
Results
In total, 47 patients had at least one anti-factor Xa concentration measured during the study period. Baseline characteristics of the patient cohort can be found in Table 1. Most of our patients were <1 year of age, which is representative of the cardiovascular surgical patient population at our centre; in 2016, 80% of patients who underwent cardiovascular surgery with cardiopulmonary bypass were <1 years. Initial anti-factor Xa concentrations are illustrated in Figure 2. In all, 71% of the initial anti-factor Xa concentrations were not within the prophylactic goal of 0.25–0.49 IU/ml, with most falling below the target range. The median initial anti-factor Xa concentration in this cohort was 0.13 IU/ml (0.06–0.19). In total, 31 patients ultimately achieved an anti-factor Xa concentration within the desired prophylactic goal range (Table 2). Younger patients were more likely to achieve in-range anti-factor Xa concentrations. Furthermore, in-range anti-factor Xa concentrations tended to be skewed towards the lower end of the prophylactic goal range and enoxaparin doses required to achieve goal concentrations were often higher than traditional prophylactic dosing recommendations.

Figure 2 Distribution of initial anti-factor Xa concentrations.
Table 1 Patient characteristics.

Data represented as median (25, 75%) or absolute count (%)
* Gestational age available for 42 of 47 patients in the study (89%)
** Race was self-reported. Respondents could report as many races as they wished
Table 2 Patients who achieved anti-factor Xa concentrations within goal range.

AFXa=anti-factor Xa
Data represented as median (25, 75%)
None of the patients in the study cohort experienced major or minor bleeding events while on prophylactic enoxaparin according to definitions created by the International Society on Thrombosis and Haemostasis.Reference Mitchell, Goldenberg and Male 16
Discussion
Central venous catheter-related thrombotic events are increasingly recognised as significant causes of morbidity in children with CHD, with reported rates as high as 25%.Reference Giglia, Massicotte and Tweddell 1 , Reference Monagle, Chan and Goldenberg 5 – Reference Hanson, Punzalan and Arca 9 Currently, however, there is no clear evidence relating to the efficacy of prophylactic anti-coagulation in preventing catheter-related thromboses in this patient population. The most recent CHEST guidelines published in 2012 and a consensus statement from the American Heart Association currently do not recommend enoxaparin for central venous catheter-related thromboprophylaxis.Reference Monagle, Chan and Goldenberg 5 Despite the paucity of data and absence of formal recommendations, many centres are concerned with the risks of central venous catheter-associated thrombosis and use prophylactic enoxaparin in children with CHD, traditionally implementing standard dosing recommendations.Reference Raffini, Trimarchi and Beliveau 8 , Reference Hanson, Punzalan and Arca 9
Diab et alReference Diab, Ramakrishnan and Ferrell 7 recently evaluated the use of intravenous versus subcutaneous enoxaparin therapy in critically ill infants and children. Although this retrospective evaluation was not limited to CHD patients and included individuals who received prophylactic and therapeutic enoxaparin, to our knowledge it is the only other evaluation to date that assesses the enoxaparin dosing requirements for children to achieve a specific prophylactic anti-factor Xa goal range. In this study, a higher percentage of patients achieved goal range (75%) using traditional dosing regimens, although the anti-factor Xa goal range used by the investigators for prophylaxis was 0.1–0.3 IU/ml, which is slightly lower than the goal range of 0.25–0.49 IU/ml in our protocol.Reference Diab, Ramakrishnan and Ferrell 7 Of note, a multicentre, prospective trial published in 2003 evaluating the efficacy of the low-molecular-weight heparin, reviparin, in the prevention of catheter-related thrombosis also targeted an anti-factor Xa concentration of 0.1–0.3 IU/ml and found no difference in the rate of catheter-related thrombosis in children.Reference Massicotte, Julian and Gent 13 Indeed, based on current literature, the prophylaxis target range of 0.1–0.3 IU/ml may be too low to yield measurable improvements in patient outcomes.Reference Massicotte, Julian and Gent 13 , Reference Schroeder, Axelrod and Silverman 14 Rather than abandon this medication as a potential effective agent against the challenging problem of catheter-related thrombosis, we believe that establishing a more aggressive goal range for anti-factor Xa concentration and the drug dosage required to attain this range were reasonable next steps.
At our institution, we aimed for the prophylactic goal range of 0.25–0.49 IU/ml and demonstrated that patients under 2 years of age with CHD required consistently higher doses of enoxaparin than are currently recommended to achieve this range. Our experience with prophylactic enoxaparin dosing is consistent with the current literature examining therapeutic enoxaparin dosing in this age group. Andrade-Campos et alReference Andrade-Campos, Montes-Limón and Fernandez-Mosteirin 17 found higher therapeutic dosing requirements in a case series (n=14) of children in which doses ranged from 1.5 to 2.7 mg/kg/dose every 12 hours for patients <1 year of age.Reference Andrade-Campos, Montes-Limón and Fernandez-Mosteirin 17 Schloemer et alReference Schloemer, Abu-Sultaneh and Hanson 11 later analysed therapeutic enoxaparin dosing in 192 critically ill children and found that they required higher than traditional dosing recommendations to achieve goal therapeutic anti-factor Xa concentrations, with only 42% achieving goal range on initial dosing – for example, 1.5 mg/kg/dose every 12 hours for patients <2 months of age and 1 mg/kg/dose every 12 hours for patients ≥2 months of age.
Importantly, we did not observe any bleeding complications in our cohort, many of whom were post-surgical, despite aiming for a slightly more aggressive prophylactic goal range than earlier reports. On the basis of our results and the absence of any noticeable side effects, we have changed the central venous catheter protocol for children with CHD at our institution to include higher empiric enoxaparin prophylaxis dosing for both age groups: 0.9 mg/kg/dose every 12 hours for infants <2 months of age and 0.75 mg/kg/dose every 12 hours for infants 2–12 months of age. We are now focusing our efforts on prospectively determining whether titration of enoxaparin therapy towards an anti-factor Xa concentration goal range of 0.25–0.49 IU/ml will result in a decreased occurrence rate of catheter-associated thrombosis and ensuring that bleeding risk associated with this more aggressive prophylactic dosing of enoxaparin is not heightened.
Conclusions
Enoxaparin doses for patients <2 years of age with CHD required to achieve prophylactic anti-factor Xa concentrations between 0.25 and 0.49 IU/ml were consistently higher than currently recommended paediatric prophylactic dosing regimens. Further study is needed to determine whether dose titration to achieve anti-Factor Xa concentrations within this goal range is an effective means of preventing central venous catheter-related thrombus formation in this patient population.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Indiana University Institutional Review Board.







