Celiac disease is an autoimmune disorder, caused by gluten and related prolamins found in foods such as wheat, barley, rye, and oats in genetically susceptible individuals. Gluten-related clinical symptoms are characterised by the presence of Celiac disease-specific antibodies, HLA-DQ2 or HLA-DQ8 haplotypes, and enteropathy. Tissue transglutaminase autoantibodies in patients with Celiac disease can affect the heart, vessel wall, and mesenchymal tissues, as well as the intestines. Reference Husby, Koletzko and Korponay-Szabó1,Reference Demirçeken2 An increased risk of cardiovascular morbidity and mortality has been reported due to cardiac effects such as ischemic heart disease, increased risk of arrhythmia, myocarditis, pericarditis, and pericardial effusion. It is also known that the prevalence of Celiac disease in patients with cardiomyopathy candidate of heart transplantation is higher than in the normal population. This effect may be due to immune response or autoantibodies. Reference Emilsson, Smith, West, Melander and Ludvigsson3,Reference Mogyorósy, Felszeghy and Kovács4 Nutritional losses, especially carnitine deficiency, have been shown to be associated with cardiomyopathy in patients with Celiac disease. It has also been reported that immune response due to tissue transglutaminase antibodies may cause early development of atherosclerosis by damage to the coronary endothelial tissue. Reference Curione, Danese and Viola5,Reference Aeschlimann and Thomazy6
As the incidence of ischemic heart disease has increased in Celiac disease, it was aimed to investigate the effect of Celiac disease on myocardial functions and aortic elasticity parameters.
Materials and methods
Thirty children between the ages of 4 and 18 years followed up in Celal Bayar University Paediatric Gastroenterology Department between 25 July, 2016 and 25 July, 2017 were included in the study, as the patient group. The age of diagnosis, onset of symptoms, gastrointestinal and extraintestinal findings of all patients were recorded. Age, gender, comorbid diseases, drug usage, and chronic diseases were recorded in all cases. Body weight and height were measured. Dietary compliance was recorded according to the history.
Thirty healthy children with negative celiac screenings who were admitted to the paediatric cardiology outpatient clinic due to other reasons (such as murmur, chest pain, syncope) were included in the study as a control group. Patient and control groups were matched in terms of age and gender.
Complete blood count, C-reactive protein, sedimentation, aspartate aminotransferase, Alanine aminotransferase, urea, creatinine, uric acid, lipid profile, electrolytes, serological anti gliadin antibody immunoglobulin A and immunoglobulin G, endomysial antibody immunoglobulin A, Anti-tissue transglutaminase immunoglobulin A antibodies (DTGA) immunoglobulin A results were recorded. Cardiac functions of all children were evaluated by conventional transthoracic echocardiography and tissue Doppler imaging. M-mode conventional transthoracic echocardiography and pulse Doppler echocardiographic examinations of the cases were performed by using a GE-Vingmed Vivid-6 system ultrasound device and a 3S transducer. Tissue Doppler evaluations were made according to the recommendations of the American Society of Echocardiography. Reference Andersen, Smiseth and Dokainish7
In both groups; for the assessment of left ventricular systolic functions; intravetricular septum thickness, left ventricular posterior wall thickness, left ventricular end diastolic diameter and end systole diameters, left ventricular ejection fraction and fractional shortening were measured via M-Mode by conventional transthoracic echocardiography. Left ventricular mass and mass index were calculated in all patients. For the assessment of left ventricular diastolic functions, early diastolic mitral annular and late diastolic mitral annular wave velocity and ratio in the mitral valve were measured by pulse Doppler. For systolic and diastolic functions, myocardial performance index was calculated.
For the tissue Doppler imaging, systolic mitral annular velocity, early diastolic mitral annular, late diastolic mitral annular, isovolumetric relaxation time, isovolumetric contraction time, ejection time, and myocardial performance index were calculated from the mitral-aortic and lateral mitral annulus.
To evaluate aortic elasticity, systolic and diastolic diameters were measured from the ascending aorta with M-mode, at a distance of 2–3 cm from the aortic valve by echocardiography. The following formula was calculated for aortic distensibility, aortic strain, and aortic stiffness. Reference Fahey, Ko and Srivastava8
– Aortic distensibility = 2 × (AOs – AOd)/(AOd) × (SBP – DBP)
– Aortic strain (%) = 100 × (AOs – AOd)/AOd
– Aortic stiffness index (β) = ln (SBP/DBP)/([AOs – AOd]/AOd)
Patients with systemic hypertension, diabetes mellitus, presence of other systemic diseases such as autoimmune thyroiditis, presence of primary valve insufficiency, genetic cardiomyopathy, presence of pregnancy-onset cardiomyopathy, patients with chronic infection or known toxin-related cardiomyopathy, structural heart disease, coronary artery disease, collagen tissue disease, haematological disease, renal/hepatic failure, malignancy, and patients who did not give their consent were excluded from the study. The study was designed as a prospective, controlled clinical trial. The approval of the Health Sciences Ethics Committee of Celal Bayar University was obtained for the study (03 August, 2016/20.478.486-290).
Statistical analysis
The data obtained from the study were recorded in the standard program named Statistical Package for Social Sciences for Windows 15.0 and evaluated. Chi-square test was used for intergroup comparison in terms of gender. Mann–Whitney U and independent samples T tests were used to compare data between groups. Statistical significance level was accepted as p <0.05.
Results
When the demographic data of the patients were evaluated, no statistical difference was found between the age, gender, height, weight, and body mass index, blood lipid levels and C-reactive protein, between the patient and control groups. Dietary compliance rate of cases in celiac group was 80% . In the celiac group, 20 patients (66.6%) had positive DTGA, 19 (63.3%) had endomysial antibody, and 13 had (43.3%) anti gliadin antibody antibodies. There was no statistical difference in systolic, diastolic blood pressure, and heart rate in both groups (p = 0.662, p = 0.759, p = 0.487), (Table 1). When conventional transthoracic echocardiography and M-mode measurements were evaluated in the patient and control groups, the intravetricular septum thickness wall thickness was found to be lower in the patient group and this was statistically different (p = 0.000). Ejection fraction and fractional shortening for systolic functions were normal and similar (p = 0.910). No statistical difference was found between E wave velocity, A wave velocity, and E/A ratio in mitral-aortic pulse Doppler evaluation (p = 0.923, p = 0.973, p = 0.785). A statistically significant difference was found between the groups in terms of isovolumetric relaxation time, isovolumetric contraction time, ejection time, and myocardial performance index in the mitral-aortic and mitral lateral annulus pulse Doppler evaluation (p = 0.000, p = 0.000, p = 0.000, p = 0.000) (Table 2). No statistical difference was found between the groups in terms of aortic strain, distensibility, and stiffness index (p = 0.690, p = 0.660, p = 0.628) (Table 3). When the DTGA immunoglobulin A, endomysial antibody immunoglobulin A, anti gliadin antibody positive and negative subgroups were compared with each other in the celiac group, there was no statistically significant difference in M-mode, pulse Doppler, and aortic elasticity parameters.
Table 1. General characteristics of patient and control group

BP = Blood Pressure
Table 2. Conventional echocardiography measurements and aortic elastic parameters of the patient and control group
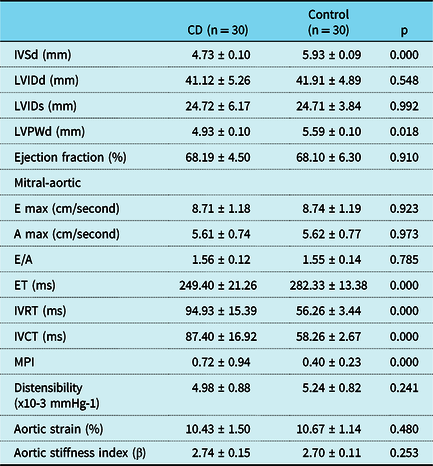
A wave velocity = Late diastolic peak flow velocity; E wave velocity = Early diastolic peak flow velocity; E/A ratio = Ratio of early and late diastolic flow velocities; Ejection fraction (%) = Ejection fraction; ET = Ejection time; IVCT = Left ventricular isovolumic contraction time; IVRT = Left ventricular isovolemic relaxation time; IVSd = İnterventricular septum enddiastolic diameter; LVEDD = Left ventricular enddistolic diameter; LVESD = Left ventricul endsystolic diameter; LVPWd = Left ventricular posterior wall enddiastolic diameter; MPI = Myocardial performance index
Table 3. Lateral mitral annulus tissue Doppler parameters of patient and control group

A wave velocity = Late diastolic peak flow velocity; E wave velocity = Early diastolic peak flow velocity; E/A ratio = Ratio of early and late diastolic flow velocities; ET = Ejection time; IVCT = Left ventricular isovolumic contraction time; IVRT = Left ventricular isovolemic relaxation time; MPI = Myocardial performance index; Sa = Systolic mitral annular velocity
Discussion
Although Celiac disease is known as an enteropathy related with autoimmune mechanisms, it has become multi-systemic disease by revealing its extra-gastrointestinal system findings in recent years. Reference Maki, Lohi, Walker, Goulet, Kleinman, Sherman, Shneider and Sanderson9 It can be asymptomatic or may present with serious symptoms. Dilated cardiomyopathy is the most common among cardiomyopathies, and it can be 50% idiopathic as it can be caused by many reasons such as genetic reasons, endocrine disorders, collagen tissue disease, drugs, structural heart diseases, and myocarditis. Reference Frustaci, Cuoco and Chimenti10 It has been reported that the incidence of Celiac disease is increased in idiopathic cardiomyopathies as well as in secondary cardiomyopathies. Reference Frustaci, Cuoco and Chimenti10 The incidence of Celiac disease has been found variable among adult cardiomyopathy patients. Curione et al reported 5.8% Celiac disease in 52 patients with dilated cardiomyopathy. Reference Curione, Barbato, De Biase, Viola, Lo Russo and Cardi11 Prati et al reported 1.9% Celiac disease in 275 patients with heart failure. Reference Prati, Bardella, Peracchi, Porretti, Scalamogna and Conte12 In the paediatric population, only few cases of Celiac disease related with myocarditis and cardiomyopathy have been reported. Reference Dogan, Peker and Cagan13,Reference Curione, Barbato, Viola, Francia, De Biase and Cucchiara14 In our study, we found no cases with dilated cardiomyopathy among whole. Ejection fraction and fractional shortening measured by conventional transthoracic echocardiography for systolic functions were normal when compared to control group. Parameters of MV E, MV A, MV E/A, which were measured for diastolic functions with conventional transthoracic echocardiography, were statistically similar between both groups. Saylan et al found similar ejection fraction and fractional shortening results with conventional transthoracic echocardiography; while MV E, MV A, and MV E/A values were found statistically different from the control group. Reference Saylan, Cevik, Tuna Kirsaclioglu, Ekici, Tosun and Ustundag15 Fath A et al also found prolongation in both left ventricular and right ventricular myocardial performance index values with tissue Doppler imaging. However, in their study, they did not find any difference between the groups in terms of conventional transthoracic echocardiography and myocardial performance index. Reference Fathy, Abo-Haded, Al-Ahmadi and El-Sonbaty16 In our study, we found that myocardial performance index and sub-parameters, which are isovolumetric relaxation time, isovolumetric contraction time, and ejection time for both systolic and diastolic functions measured by conventional transthoracic echocardiography and tissue Doppler imaging, were statistically different in the Celiac disease group compared to the control group. Elongation of isovolumetric contraction time and shortening of ejection time measured by both conventional transthoracic echocardiography and tissue Doppler imaging showed systolic dysfunction, and prolongation in isovolumetric relaxation time with both methods showed diastolic dysfunction. Thus, our study showed that both sub-clinic systolic and diastolic cardiac dysfunction were present in Celiac disease and started in early childhood. Young adults with Celiac disease are potentially at increased risk of atherosclerosis due to underlying vascular and biochemical disorders. Reference De Marchi, Chiarioni, Prior and Arosio17 Most population-based studies showed that patients with Celiac disease have an increased risk of cardiovascular disease compared with the normal population. Reference Wei, Spiers, Reynolds, Walsh, Fahey and MaÇHonald18 However, most of the cardiovascular events in these patients could not be explained by traditional risk factors. Emilsson et al showed that Celiac disease patients with a history of myocardial infarction had a better risk profile in terms of traditional cardiovascular risk factors compared to those without. Reference Emilsson, Carlsson, Holmqvist, James and Ludvigsson19 Therefore, it is important to identify new risk factors associated with increased cardiovascular risk in Celiac disease. In our study, we compared the groups with and without DTGA and endomysial antibody antibody positivity in children with Celiac disease, and investigated whether there would be a new cardiovascular risk factor in children with continued antibody positivity, but we could not find a significant difference between the two subgroups. We found that the antibodies circulating in the blood did not affect cardiac parameters alone. This may be due to the insufficient number of our patients. It is known that increased aortic stiffness and chronic systemic inflammation are associated with increased cardiovascular risk in different studies. Reference Yingchoncharoen, Limpijankit, Jongjirasiri, Laothamatas, Yamwong and Sritara20,Reference Tsuchikura, Shoji and Kimoto21 It has been reported that increased cardiovascular risk continues with chronic inflammation and chronic inflammation does not affect aortic parameters alone and is multifactorial in cases with Celiac disease who do not comply with gluten-free diet and whose antibody positivity continues. Reference Bayar, Çekin and Arslan22
In our study, we found no significant change in aortic elasticity parameters as an unconventional risk factor in patients with Celiac disease. This may be related to the fact that chronic systemic inflammation may have decreased with diet in these patients or that chronic inflammation in the paediatric patient group may not affect the aortic parameters alone. When we grouped children with Celiac disease as positive and negative antibody titers, we could not find a difference again. We thought that we could find this difference among subgroups with a higher number of patients.
In conclusion, although cardiomyopathies associated with Celiac disease are not common in early childhood, they should be examined in the etiology because they are serious and lethal. Identifying and defining chronic inflammation that begins in early childhood and other possible risk factors and early theropathic approaches can offer or prevent new treatment options for cardiac events that begin in paediatric patients with Celiac disease, as shown in our study.
Acknowledgements
The authors would like to acknowledge the Beyhan Cengiz Ozyurt with the statistical analysis performed.
Conflict of interest
The submission is with the full knowledge and approval of the listed authors. None of the authors have any disclosures or conflicts of interest to report.
Financial disclosure
The study has received no financial support.
Author contributions
Fatos Alkan: Study concept, study design, resource, material getting, data collection, literature search
Guzıde Dogan : Study concept, resource
Erhun Kasırga: Supervision, critical reviews
Senol Coskun: Study design, data analysis and interpretation






