Truncus arteriosus or common arterial trunk is a rare congenital cardiac malformation in which a single arterial trunk gives rise to the aorta, pulmonary arteries, and coronary arteries.Reference Lev and Saphir 1 It occurs in 1–4% of all cases of congenital heart disease; without intervention, it is typically fatal during the first year of life. Diagnosis of truncus arteriosus can be readily determined by echocardiography. Morphology and nomenclature of common arterial trunk have been discussed elsewhere in this supplement. This section will focus on key components of diagnostic imaging by two-dimensional echocardiography in neonates suspected to have truncus arteriosus. In addition, technical aspects of intra-operative transesophageal echocardiography and follow-up echocardiogram are discussed.
Neonatal pre-operative evaluation
In the newborn period, echocardiography remains the primary, and in most cases the only, mode of evaluation before surgical repair. Therefore, a detailed assessment by echocardiography is critical; it should thoroughly evaluate all components of anatomic features by segmental approach and using multiple imaging views, taking care to identify the usual and unusual cardiac anomalies associated with truncus arteriosus.
Objectives of the first echocardiogram in the newborn period:
-
• Identify the location and extent of the ventricular septal defect, and the presence of any additional ventricular septal defect(s).
-
• Evaluate the morphology and function of the atrioventricular valves, particularly identifying any straddling chordae or valve tissue across the ventricular septal defect.
-
• Determine the morphology and function of the truncal valve.
-
• Evaluate pulmonary artery anatomy. Determine the presence or absence of the main pulmonary artery. Describe origin(s) and size of the branch pulmonary arteries.
-
• Evaluate additional sources of pulmonary blood flow, such as ductal artery and aortopulmonary collaterals, which are especially important in the setting of interruption of the aorta or abnormal origin of one of the branch pulmonary arteries not arising from the ascending aorta.
-
• Evaluate the aortic arch for sidedness, coarctation, or interruption. Right aortic arch occurs in approximately 33% of patients.Reference Calder, Van Praagh and Van Praagh 2 Interrupted aortic arch occurs in 19%.Reference Van Mierop and Kutsche 3
-
• Evaluate ventricular size and function.
-
• Describe coronary artery anatomy and distribution, particularly the coronary origin(s) in relationship to the pulmonary arteries and truncal valve leaflets. Single origin of the coronary artery (13–18%) with intramural course should be identified.
-
• Evaluate for any other associated lesions including the presence of atrial septal defect (10–20%), left superior vena cava to coronary sinus (5–15%), anomalous pulmonary venous connection (1%). 4
Subcostal view
Subcostal imaging gives the most complete view of the anatomy. It is usually the preferred initial imaging approach in many centres. Cardiac position and segmental connections can be easily established at the beginning of the evaluation with a good sweep in the long axis of subcostal view. At the level of the diaphragm, the hepatic veins come together with the inferior caval vein to drain into the right atrium. The transducer is tilted towards the cardiac base, and hence the coronary sinus, atria, and ventricles come readily into view. A dilated coronary sinus should alert the echocardiographer to the possibility of a persistent left superior caval vein. At the level of the four-chamber cut, the atrial septum can be assessed and the pulmonary veins can be seen draining into the left atrium. The inlets of the heart and the sizes of the ventricular chambers can be assessed. Owing to the fact that the sweep continues superiorly, the location and extent of the ventricular septal defect and relationship of the common trunk to the ventricles can be determined. With a slight anterosuperior angulation, the truncal valve overriding the ventricular septal defect can be imaged. The branch pulmonary arteries and their origins from the common trunk should be well visualised. The main pulmonary artery, if present, is often seen arising from the left and posterior aspect of the common trunk (Fig 1a). With clockwise rotation of the transducer to the short-axis view, the outlet of the right ventricle could be assessed. The right pulmonary artery can be seen just inferior and posterior to the arterial trunk (Fig 1b).
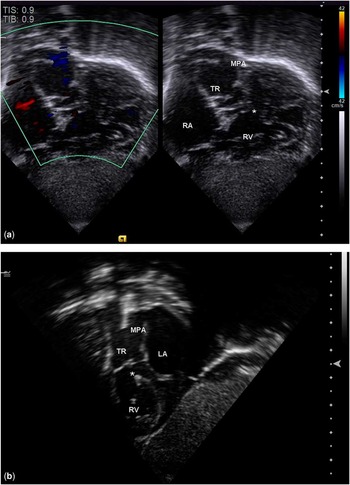
Figure 1 Long-axis ( a ) and short-axis ( b ) of the subcostal view demonstrating the posterior and leftward location of the main pulmonary artery from the truncal root. The truncal valve is also seen overriding the ventricular septal defect (*). LA = left atrium; MPA = main pulmonary artery; RA = right atrium; RV = right ventricle, TR = truncal root.
Parasternal view
The parasternal views are useful for evaluation of the morphology and function of the truncal valve, coronary and pulmonary artery anatomy, extent of the ventricular septal defect, and ventricular size and function. In the parasternal long-axis view, a single arterial trunk arising from the base of the heart can be seen overriding the ventricular septum in the presence of a malalignment type of ventricular septal defect. There is fibrous continuity between the mitral valve and the truncal valve. As the transducer is swept to the patient's left, the short main pulmonary artery can be seen arising from the posterior aspect of the truncus (Fig 2). An enlarged coronary sinus in this view also suggests the presence of a persistent left superior vena cava. In parasternal short-axis view, the truncal valve can be well visualised. The morphology, commissures, and number of leaflets should be evaluated and documented. The leaflets are often thickened and often described as nodular. The most common morphology is trileaflet, occurring in up to 65–70% of patients, with quadricuspid in 9–24%, bicuspid in 6–23%, and rarely unicuspid. (Fig 3a and b). Truncal valve insufficiency can be assessed using colour Doppler. The mechanism of truncal regurgitation should be sought. The use of three-dimensional echocardiography can also provide additional insight into valvar morphology and function. Parasternal views are not often used to assess the severity of truncal stenosis because of poor angle of Doppler interrogation to the direction of flow across the valve. However, truncal valve stenosis can be suggested by the appearance of thickened leaflets with poor excursion. Parasternal short-axis view is an excellent view to determine the type of truncus arteriosus by evaluating the anatomy of the branch pulmonary arteries. With the transducer angled superiorly above the level of the truncal valve, the origins of the branch pulmonary arteries can be seen arising along the posterior aspect of the common trunk either from a common orifice or separate orifices (usually closely spaced; Fig 4a and b). When the origins of the branch pulmonary arteries are widely spaced, it is seldom possible to image both branch pulmonary arteries in the same plane. The coronary artery origins and proximal courses are usually best imaged in short-axis view at the level of the truncal valve with slight clockwise rotation of the transducer. The right coronary artery usually originates from the anterolateral aspect and the left coronary artery from the left posterolateral aspect of the truncal root. Spatial relationships of the coronaries to the truncal valve and pulmonary arteries need to be described clearly because of important implications in surgical repair. The presence of single coronary artery and/or intramural course needs to be identified (Fig 5). The presence of coronary arteries in the infundibular free wall may also complicate the placement of the right ventricle to pulmonary artery conduit as part of the surgical repair. The extent and size of the ventricular septal defect, presence of additional ventricular septal defect(s), and ventricular function can be thoroughly assessed as the transducer is swept to the apex of the heart.
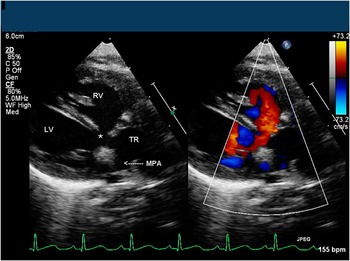
Figure 2 Parasternal long-axis imaging plane demonstrating truncal override of the ventricles and the pulmonary artery origin from the TR. LV = left ventricle; MPA = main pulmonary artery; RV = right ventricle; TR = truncal root; *, ventricular septal defect.

Figure 3 Parasternal short-axis imaging plane demonstrating morphology of the truncal valve: quadricuspid ( a ) and unicuspid ( b ).

Figure 4 ( a ) Parasternal short-axis imaging plane with colour Doppler interrogation demonstrating common orifice of the pulmonary arteries from the TR. The branch pulmonary arteries appear to be of good size without discrete narrowing stenosis seen. ( b ) Parasternal short-axis imaging plane showing separate orifices of the pulmonary artery branches from the TR. LPA = left pulmonary artery; RPA = right pulmonary artery; TR = truncal root.

Figure 5 Parasternal short-axis imaging plane showing abnormal origin of the left coronary artery from the RCA. The left coronary artery appears to originate from the right side and courses posterior to the TR. LCA = left coronary artery; RCA = right coronary artery; TR = truncal root.
Apical view
The apical four-chamber view is useful for evaluation of the atrioventricular valves, function of the truncal valve, ventricular septum, ventricular sizes, and origin of the main and/or branch pulmonary arteries. Sizes and function of the tricuspid and mitral valves can be evaluated with the aid of spectral and colour Doppler. Owing to the fact that the transducer is swept anteriorly, the typical malalignment ventricular septal defect with an overriding aorta is seen. Any straddling of the chordae of valve tissue across the ventricular septal defect can be identified during the sweep. The branch pulmonary arteries and their origin(s) from the common trunk can usually be tracked to the posterior aspect of the truncal root as the transducer is tilted anteriorly (Fig 6). The apical view is also useful for assessing truncal stenosis and insufficiency due to the fact that the Doppler beam can be aligned parallel to the flow. Accurate Doppler gradient can be obtained when stenosis is present. Peak and mean spectral Doppler gradients should be obtained to estimate the severity of the truncal valve stenosis. In addition, regurgitant jet characteristics and Doppler velocity pressure half-time can be used to assess the degree of truncal valve regurgitation.

Figure 6 Apical imaging plane showing the origin of the main pulmonary from the TR. Flow into the MPA appears unobstructed. LPA = left pulmonary artery; MPA = main pulmonary artery; RA = right atrium; RPA = right pulmonary artery; TR = truncal root.
Suprasternal notch view
The suprasternal notch view is most helpful in identifying arch sidedness, coarctation, and interruption of the aortic arch. The right aortic arch has been found in approximately one-third of patients with truncus arteriosus. 4 In addition, interrupted aortic arch occurs in ∼19% of patients with truncus arteriosus. The suprasternal notch is the best window to image the aortic arch (Fig 7). In this view, interruption of the aortic arch is suspected when a ductal arch is seen supplying the descending aorta. With the transducer angling to the left of the patient in the sagittal plane, the ductal arch can be seen as a separate structure from the aortic arch. Interruption in truncus arteriosus is usually of type B, in which the left subclavian artery arises from the descending aorta with flow filled from the ductal arch. Carotid arteries arise from the aortic arch. Doppler interrogation of the descending aorta usually demonstrates typical diastolic flow reversal pattern. This may be due to diastolic flow into the pulmonary arteries and/or presence of significant truncal valve insufficiency.
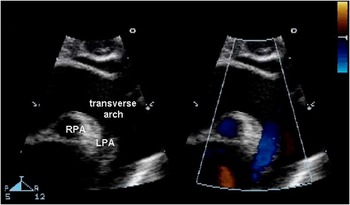
Figure 7 Suprasternal notch long-axis imaging plane demonstrating a patent aortic arch with abnormal origin of the LPA from the descending aorta. The RPA is seen in cross-section originating from the TR, whereas the LPA originates from the underside of the aortic arch as clearly illustrated by colour flow Doppler. LPA = left pulmonary artery; RPA = right pulmonary artery; TR = truncal root.
Intra-operative assessment
This section will focus on the intra-operative transesophageal evaluation of truncus arteriosus, which consists of the pre-operative and post-operative transesophageal echocardiograms. The pre-operative transesophageal echocardiogram should primarily be concerned with confirmation of the findings of previous transthoracic echocardiograms. The post-operative transesophageal echocardiogram should focus on the assessment of the effectiveness of the surgical repair of truncus arteriosus and evaluate for any residual defects or ventricular dysfunction.
For general indications and guidelines regarding paediatric transesophageal echocardiogram, the reader should refer to the previously published article by the Task Force of the Pediatric Council of the American Society of Echocardiography.Reference Ayeres, Miller-Hance and Fyfe 5 This article addresses paediatric transesophageal echocardiogram indications, contraindications, complications, limitations, safety issues, subacute bacterial endocarditis prophylaxis, and even training and certification guidelines. With regard to the limitations of transesophageal echocardiographic imaging, the cardiologist should be aware that transesophageal echocardiogram does not adequately visualise the transverse arch and isthmus, and often cannot completely assess the branch pulmonary arteries. Complete pre-operative assessment of the aortic arch and branch pulmonary arteries is critical in truncus arteriosus. If these structures cannot be completely delineated by transthoracic echocardiography, one should consider alternative imaging such as magnetic resonance imaging, non-gated computed tomography, or diagnostic catheterisation.
One of the advantages of transesophageal echocardiography is the infinite variety of imaging planes. With modern paediatric multi-plane probes, the operator has 180° of scanning angle. In addition, the probe may be manipulated with insertion, withdrawal, tip flexion and extension, as well as probe rotation. These numerous possible imaging planes necessitate that one have “a careful, detailed and consistent systematic approach to each and every transesophageal echocardiographic examination performed”.Reference Ayeres, Miller-Hance and Fyfe 5 It is critical that this detailed and complete systematic approach be applied to both pre-operative and post-operative transesophageal echocardiograms. The post-operative transesophgeal echocardiogram should not be considered a “leak and function” evaluation, but should be as complete and thorough as the pre-operative transesophageal echocardiogram.
Although there are an infinite variety of possible imaging planes, for simplicity of discussion in this article we will address the most common imaging planes in paediatric transesophageal echocardiography: four-chamber view, bicaval view, aortic/pulmonary valve long-axis views, aortic valve short-axis view, right ventricular inflow/outflow view, and transgastric views.
Four-chamber view
Institutional and personal practices vary, but many recommend that each transesophageal echocardiogram begins with a complete four-chamber view sweep, starting with full insertion in order to image the posterior structures including coronary sinus and atrioventricular valves, then withdrawing while continually recording, and concluding with views of the proximal great arteries. The scanning angle is usually 0° for the four-chamber view. The four-chamber view allows assessment of atrial septal defect(s), ventricular septal defect(s), tricuspid valve, mitral valve, pulmonary veins, chamber size, and ventricular function. When assessing truncus arteriosus for additional ventricular septal defect(s) or residual ventricular septal defect(s), one should lower the Nyquist limit and sweep the entire ventricular septum by inserting and withdrawing the probe. A lower Nyquist limit should also be used when assessing the left and right pulmonary veins. A dilated coronary sinus suggests persistent left superior vena cava, which occurs in 12% of patients with truncus arteriosus.
Bicaval view
When performed in conjunction with the four-chamber view, the bicaval view allows orthogonal interrogation of the atrial septum. The usual scanning angle is 90°. This view also allows excellent assessment of the superior vena cava and inferior vena cava. With slight rightward/clockwise rotation, one can also visualise the right upper pulmonary vein.
Aortic/pulmonary valve long-axis views
In truncus arteriosus, a scanning angle of ∼120° provides the aortic/pulmonary valve long-axis views, which allow two-dimensional and colour Doppler visualisation of the truncal valve and ascending aorta. This is often the best view to visualise truncal regurgitation. With rotation, one can often visualise the proximal branch pulmonary arteries and their origins from the ascending aorta. Owing to the fact that the ventricular septal defect in common arterial trunk is usually perimembranous, it should be well seen in this view. After complete repair, residual truncal regurgitation, the right ventricle to pulmonary artery conduit, and any residual ventricular septal defect of the perimembranous region should all be seen with the aortic/pulmonary valve long-axis views. Owing to the angle of interrogation, this view is not suggested for Doppler interrogation of the truncal valve.
Aortic valve short-axis view
Truncal valve morphology is best seen from the aortic valve short-axis view (Fig 4). An assessment of truncal valve morphology should include not only the number of leaflets, but also a description of those leaflets – thickened, dysplastic, doming, redundant, prolapsing, etc. With appropriate magnification and lowering of the Nyquist limit, the proximal coronary arteries should be visualised in the truncal valve short-axis view. The location of a truncal regurgitation jet can also be assessed with this view. To achieve a short-axis view of the truncal valve, a scanning angle between 25° and 50° is usually necessary.
Right ventricular inflow/outflow view
This view is especially useful after complete repair. It allows simultaneous assessment of the tricuspid valve, systolic function of the right ventricle, and proximal right ventricular to pulmonary artery conduit, including the conduit valve if one is present. With appropriate probe withdrawal and rotation, the anastomosis between the conduit and right pulmonary artery is demonstrated (Fig 8). The left pulmonary artery is often not well seen by transesophageal echocardiogram. Residual ventricular septal defects can also be detected with this view. The required scanning angle is ∼60°.
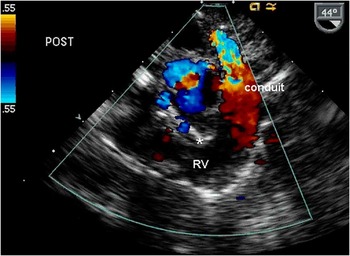
Figure 8 Transesophageal imaging plane showing inflow and outflow of the RV following complete repair. Colour Doppler demonstrates flow acceleration across the RV to pulmonary artery conduit. A ventricular septal defect patch (*) can also be seen. RV = right ventricle.
Transgastric views
Owing to their near-optimal angle of interrogation, the transgastric views are the best views for spectral Doppler interrogation of the truncal valve. After repair, the view also offers a reasonable angle of interrogation for spectral Doppler assessment of the right ventricular to pulmonary artery conduit valve. To achieve the transgastric views, unlock the probe tip and advance it into the stomach. Then use slight tip flexion while slowly withdrawing. Slight adjustments in probe rotation and scanning angle are necessary to achieve the best images. The usual scanning angle is around 0° for the truncal valve and 60° for the conduit.
The key to performing a complete transesophageal echocardiogram on a patient with truncus arteriosus is actually the pre-existing knowledge of what you are looking for and what you can expect to find. One must know what possible undetected defects to expect on the pre-operative transesophageal echocardiogram, and what possible residual defects are likely on the post-operative evaluation. The cardiologist must also be aware of what defects cannot be well seen by transesophageal echocardiogram. Finally, one must apply a detailed and complete systematic approach to both pre-operative and post-operative transesophageal echocardiograms.
Follow-up echocardiogram
Non-invasive imaging with transthoracic echocardiography plays an integral role in the post-operative and long-term management of patients with common arterial trunk. Surgical repair typically involves removing the pulmonary arteries from the common trunk, closing the ventricular septal defect, and inserting a conduit between the right ventricle and the pulmonary arteries. The truncal valve may also require repair or replacement in situations where there is significant stenosis or regurgitation. After initial surgical correction, patients will require replacement of their right ventricle to pulmonary artery conduit. Following surgical repair, patients require serial assessment of several aspects of the repair.
Goals of follow-up or serial echocardiograms should include the following:
-
• evaluate for any residual ventricular septal defect;
-
• evaluate right ventricle to pulmonary artery conduit;
-
• evaluate branch pulmonary arteries;
-
• assess for serial changes in the function of the neo-aortic or truncal valve;
-
• evaluate aortic arch, in cases of associated interrupted aortic arch;
-
• determine right ventricular systolic pressure; and
-
• evaluate right and left ventricular size and function.
The presence of a residual ventricular septal defect is possible after surgical repair. Size and flow velocity through a residual ventricular septal defect can be analysed. A residual outlet defect is best viewed from the parasternal short-axis view at the base of the heart with the addition of colour Doppler. The apical and subcostal views can also aid in the evaluation of residual ventricular septal defects.
The integrity of the right ventricle to pulmonary artery conduit must be serially assessed. These conduits can become stenotic and/or regurgitant with time. In addition, these conduits must be replaced because of somatic growth of the patient. The peak spectral Doppler velocity and pressure gradient across the conduit is obtained in order to assess the degree of stenosis. The right ventricular systolic pressure can be determined with the peak Doppler velocity across either a residual ventricular septal defect or tricuspid regurgitation jet.
The development of pulmonary artery branch stenosis is also known to occur. In addition, there can be pulmonary artery branch hypoplasia. The pulmonary artery branches are best interrogated in the parasternal short-axis and suprasternal views. Both colour and spectral Doppler are used in determining the presence of branch stenosis.
The neoaortic or truncal valve may become regurgitant or stenotic over time and must be evaluated serially. Apical and subcostal views aid in assessment. In the case of a stenotic valve, there may be limited excursion of the valve leaflets and/or thickened valve leaflets appreciated by two dimensional. Turbulent flow or aliasing by colour Doppler suggests stenosis. From the apical view, an accurate gradient can be obtained because of parallel alignment of the ultrasound beam with the truncal valve. Continuous wave Doppler and the Bernoulli equation can calculate peak and mean gradients across the valve. Left ventricular hypertrophy and function should also be interrogated when neoaortic or truncal valve stenosis is present. The presence of diastolic flow reversal in the descending aorta, assessment of left ventricular dilation and function, and obtainment of a pressure half-time measurement can assist in determining the severity of regurgitation.
Summary
Echocardiography provides accurate assessment of the cardiac anatomy in truncus arteriosus. It remains the primary mode of pre-operative and intra-operative evaluation, as well as ongoing monitoring in this population of patients.










