Coarctation of the aorta
Definition
Coarctation of the aorta is defined as congenital narrowing of the aorta, which typically occurs at the aortic isthmus, but can occur anywhere along the thoracic or abdominal aorta. This may be associated with concomitant hypoplasia of the aortic arch.
Classification
Historically, there have been several classification schemes for coarctation of the aorta. BonnetReference Bonnet 2 in 1903 classified coarctation of the aorta into two types: infantile – “preductal” – and adult – “postductal” – owing to the significant pathophysiological differences between an adult with chronic coarctation of the aorta and an infant with duct-dependent coarctation of the aorta. Van Praagh et alReference Van Praagh, O’Connor and Chacko 3 raised the concern that classification of coarctation of the aorta in relationship to the position of the arterial duct was inaccurate, as the site of coarctation is almost always juxtaductal or at the level of the arterial duct. Amanto et alReference Amanto, Galdieri and Cotroneo 4 proposed a classification of coarctation of the aorta that incorporated the concept of hypoplasia of the aortic arch, as this modifies the surgical repair of this lesion. His classification system is described in Table 1.
Table 1 Classification system of coarctation of the aorta as proposed by Amanto et al.Reference Amanto, Galdieri and Cotroneo 4

Options for surgical repair range from end-to-end anastomosis to extended arch reconstruction, depending on the extent of hypoplasia of the aortic arch. The surgical approaches for these two entities differ significantly – lateral thoracotomy versus sternotomy with bypass – and therefore it is important to differentiate discrete coarctation of the aorta from arch hypoplasia.
Nomenclature of arch segments
Lopez et alReference Lopez, Colan, Frommelt, Ensing, Kendall, Younoszai, Lai and Geva 5 in the American Society of Echocardiography’s guidelines for quantification methods during performance of a paediatric echocardiogram defined aortic arch segments as follows:
-
∙ proximal transverse arch – segment of the aortic arch between the innominate artery and the left common carotid artery,
-
∙ distal transverse arch – segment of the aortic arch between the left common carotid artery and the left subclavian arteries,
-
∙ aortic isthmus – narrowest aortic arch segment distal to the left subclavian artery.
This nomenclature of the segments of the aortic arch was illustrated using an echocardiogram image in the previous review article by Parthiban and Shirali.Reference Parthiban and Shirali 6 These guidelines for nomenclature are applicable in patients with left aortic arch with normal branching pattern; however, patients with coarctation of the aorta can have abnormal arch-sidedness and branching pattern. In such situations, the above classification scheme has some limitations – for example, a patient with a left aortic arch and common brachiocephalic trunk would not have a proximal transverse arch segment. Similarly, in a patient with an aberrant subclavian artery, the coarctation is typically located proximal to the aberrant vessel, and therefore the “isthmus” is also located proximal to the aberrant subclavian artery. A right-sided aortic arch presents another interesting challenge to the classification system. In practice, if the arch branching is the mirror image of the usual arrangement, then the mirror image of the usual nomenclature system is utilised with the left innominate artery, the right carotid artery, and the right subclavian arteries dividing the segments of the aortic arch.
There are published normative data in the paediatric population for diameters of each of these segments of the aortic arch.Reference Cantinotti, Scalese and Murzi 7 , Reference Pettersen, Du, Skerens and Humes 8 There are also normative data for children with Turner syndrome; they constitute a special population with a high incidence of coarctation of the aorta and lower linear growth compared with the general population.Reference Quezada, Lapidus, Shauqunessy, Chen and Silberbach 9
Imaging goals
The goals of imaging in obstruction of the aortic arch are described in Table 2. These objectives are primarily directed towards preoperative planning and confirmation of the diagnosis. Specifically, it is imperative to identify associated regions, as the extent of hypoplasia determines the type and extent of surgical repair. It is also important to evaluate the intracardiac anatomy to evaluate for associated cardiac abnormalities.
Table 2 Goals of imaging for coarctation of the aorta.

Technical tips
Echocardiography is the primary imaging modality utilised to image the aortic arch. The aortic arch is best imaged from the suprasternal notch view. This sweep should begin with the transducer notch at 3 o’clock, so that the ascending aorta is visualised in the short axis. Then a superior sweep is performed for determination of arch branching and sidedness. The transducer is then rotated to display the long-axis or “candy-cane” view of the aortic arch. In neonates, the right infraclavicular area can often demonstrate the sagittal view of the entire thoracic aorta in one plane. Occasionally, a high parasternal short-axis view, also called the “ductal view”, is required to visualise the aortic isthmus if the arch is tortuous or if the coarctation is severe. A rolled towel placed underneath the patient’s shoulders to allow for extension of the neck and upper chest is often helpful; one should use the highest frequency transducer that provides adequate penetration to visualise structures, typically 12 MHz in infants. This allows for optimal spatial resolution. The smaller footprint of a higher frequency transducer allows a better fit in the suprasternal notch. It is important to decrease the imaging depth to the minimum that is needed to view the region of interest. The transmit focus should be adjusted to electronically maintain parallel beams and avoid interpolation throughout the areas of interest. This will allow for standardised and accurate measurements.
Measurements of the segments of the aortic arch should be performed from inner edge-to-inner edge on two-dimensional images in systole. These measurements should be used to report z-scores adjusted for body surface to determine relative size of the segments of the aorta compared with the general population. The use of images with color Doppler can lead to an overestimation of size.
Imaging of the aortic arch by echocardiography can be challenging because of the presence of overlying structures such as lung segments and airway that can limit echocardiographic windows. When imaging by echocardiography is not adequate for preoperative evaluation using the above techniques, CT or MRI angiography can be helpful in demonstrating the anatomy more clearly; however, as these techniques involve exposure of the patient to contrast, radiation, and anaesthesia, these advanced modalities should be reserved for patients only when echocardiography fails.
Imaging neonatal coarctation of the aorta
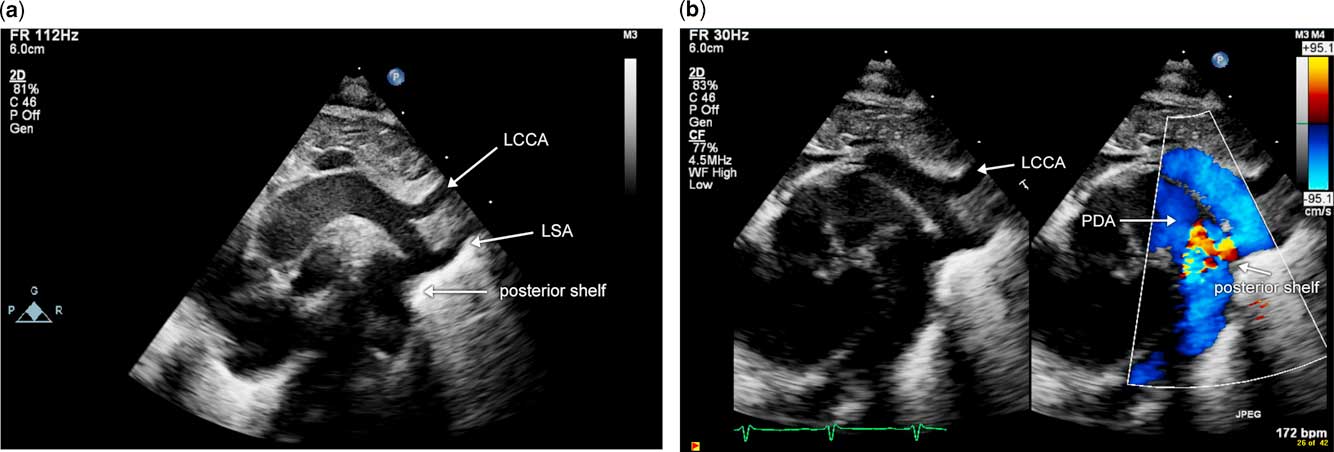
Coarctation of the aorta in the neonate can be either discrete or can occur in conjunction with aortic arch hypoplasia. Discrete coarctation of the aorta is defined as discrete narrowing in a region of the aortic arch. The discrete narrowing typically occurs in the distal arch, near the site of insertion of the arterial duct. The remaining segments of the aortic arch measure in the normal range with normal z-scores. This lesion can typically be diagnosed adequately in the neonate using transthoracic echocardiography. It can be challenging to diagnose this entity in the presence of a large arterial duct. Key features of diagnosis are the presence of a posterior shelf and hypoplasia of the aortic isthmus, both of which are determined on two-dimensional sagittal arch images (Fig 1a and b). Doppler evaluation of the aortic arch is misleading in the presence of an arterial duct, and cannot be used to either rule out or confirm a diagnosis of coarctation of the aorta. This is because flow through the arterial duct results in addition or removal of flow through the aortic isthmus, which is the region of interest. Figure 2 illustrates this concept in a neonate with critical coarctation of the aorta with a large arterial duct and normal Doppler velocities across the distal aortic arch and isthmus. The diagnosis of discrete coarctation of the aorta in the absence of an arterial duct is made more easily, and changes in flow across the aortic arch are appreciated on Doppler evaluation of the aortic arch, as illustrated in Figure 3. Again, by two-dimensional imaging, a posterior shelf and narrowing of the aortic isthmus are appreciated. With color flow Doppler, however, aliasing in the aortic isthmus indicates flow acceleration (Fig 3a). There is increased systolic flow velocity with continuation of flow in diastole – that is, the diastolic runoff pattern (Fig 3b). In addition, interrogation of the abdominal aorta using pulsed wave Doppler exhibits blunted pulsatility (Fig 3c). Surgical repair of this entity can be performed via lateral thoracotomy, and an end-to-end anastomosis can be performed.
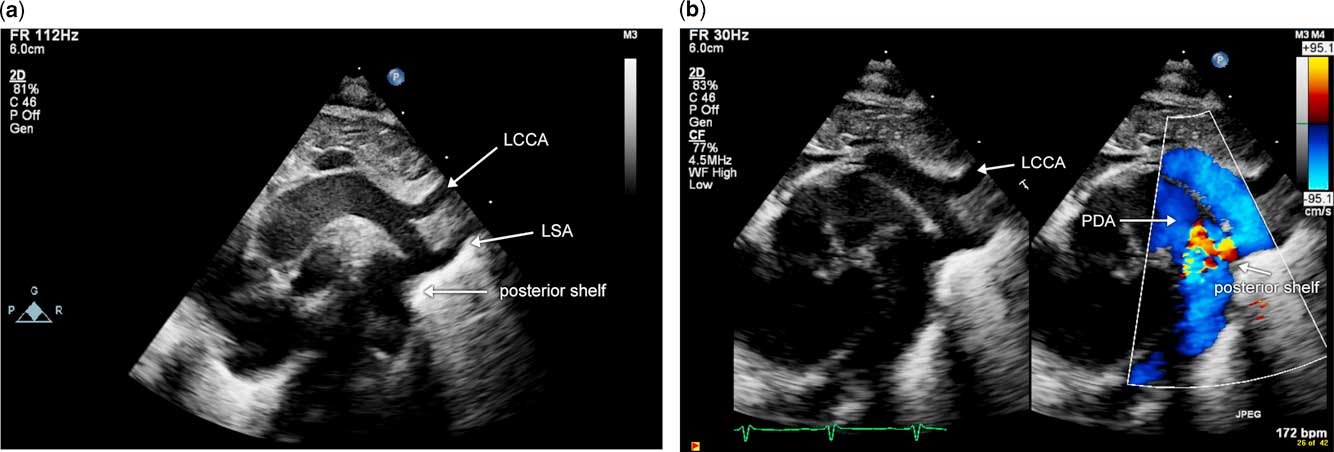
Figure 1 Image of discrete coarctation of the aorta in the presence of a large arterial duct. Note the posterior shelf, indicated by the white arrow, and isthmal narrowing just proximal to this. The arterial duct is seen anterior to the posterior shelf. ( a ) Two-dimensional echocardiographic image; ( b ) image from the same patient with color Doppler. Note colour aliasing in the region of narrowing and posterior shelf. LCCA=left common carotid artery; LSA=left subclavian artery; PDA=patent arterial duct.
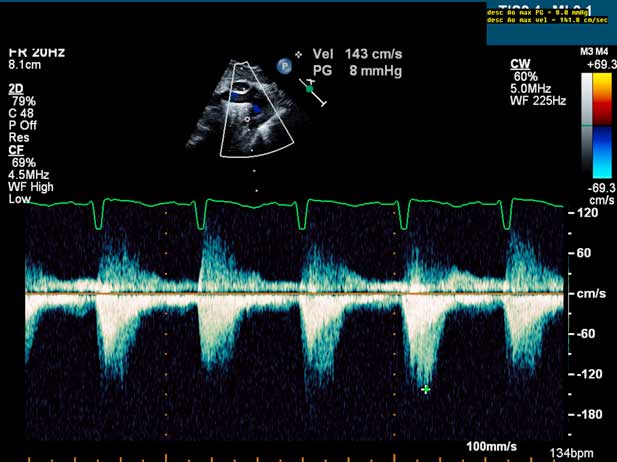
Figure 2 Pulse Doppler evaluation of coarctation of the aorta in the presence of an arterial duct in a neonate. Note the lack of flow acceleration at the aortic isthmus due to the widely patent arterial duct.
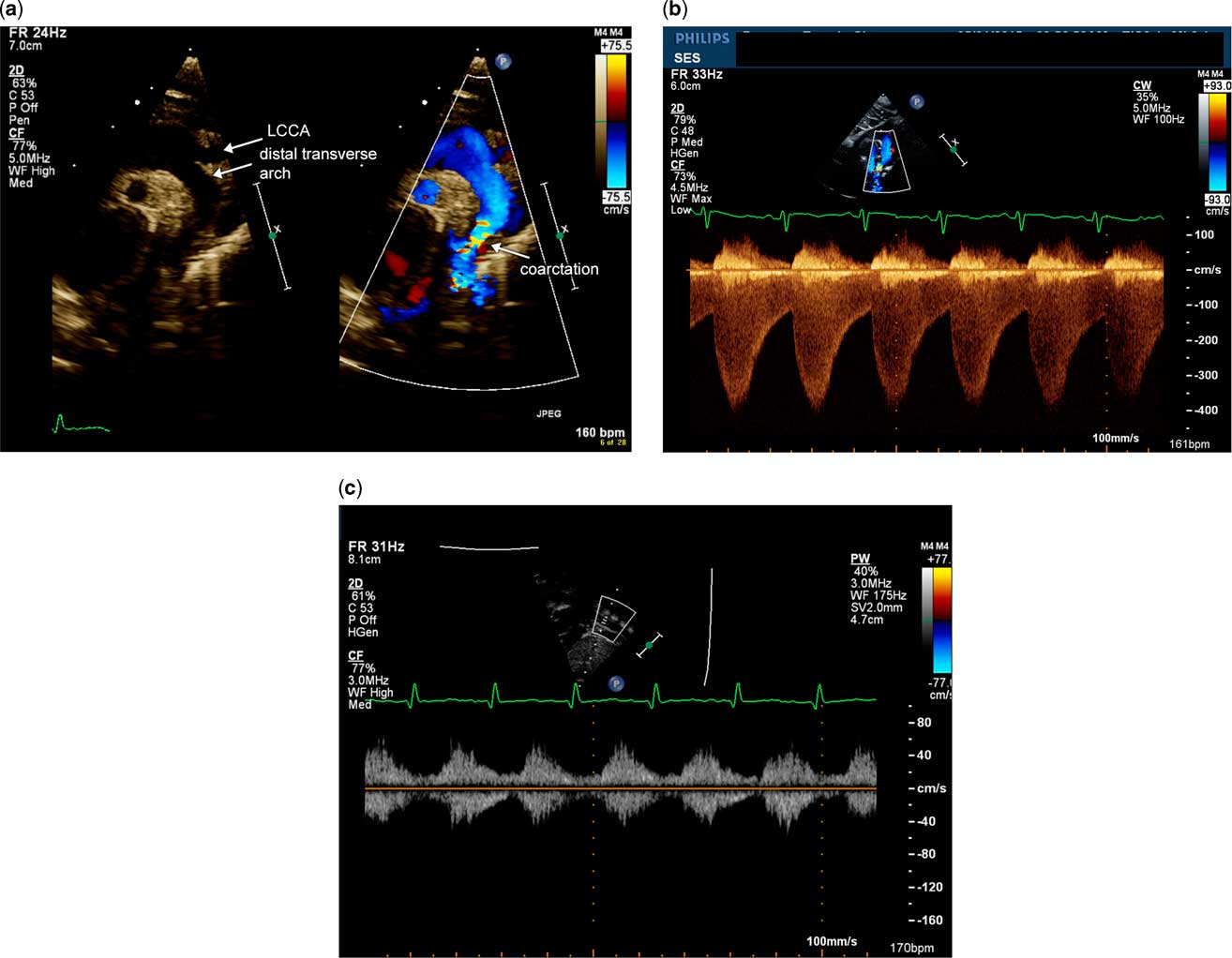
Figure 3 Discrete coarctation of the aorta in a neonate without an arterial duct. ( a ) Echocardiographic image demonstrates a sagittal view of the aortic arch with and without color flow Doppler. There is narrowing of the aortic isthmus with a posterior shelf. ( b ) Doppler evaluation of the aortic arch using continuous wave Doppler illustrates increased flow velocity in systole and runoff in diastole, typical of severe coarctation of the aorta. ( c ) Pulsed wave Doppler evaluation of the abdominal descending aorta in the subxiphoid short-axis view with notch at 6 o'clock. This Doppler has blunted pulsatility due to the severity of coarctation of the aorta. LCCA=left common carotid artery.
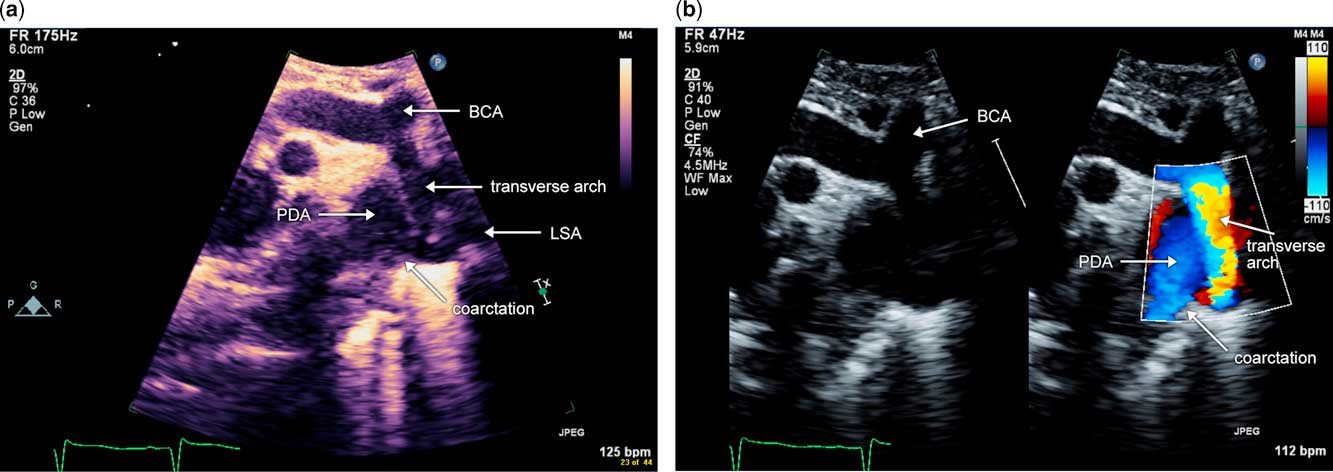
Coarctation of the aorta can occur in combination with hypoplasia of the aortic arch. In addition to narrowing and hypoplasia of the aortic isthmus with a posterior shelf, segments of the transverse arch are also hypoplastic, with z-scores of −3 or lower. Typically, in these cases, the distal transverse arch is long and hypoplastic, and the proximal transverse arch is short or non-existent, as in the case of a common brachiocephalic trunk. Surgical repair for this lesion is quite different from discrete coarctation of the aorta, and involves reconstruction of the aortic arch on cardiopulmonary bypass via median sternotomy. Doppler interrogation of the aortic arch in this anatomy is challenging as there are multiple levels of obstruction. Therefore, when evaluating coarctation of the aorta with hypoplasia of the aortic arch by echocardiography, in addition to two-dimensional imaging of the aortic arch with measurements of each segment, pulsed wave Doppler interrogation should be performed within each segment of the aortic arch. The proximal velocity should be accounted for when calculating the pressure gradient across each segment. Fig 4a and b are echocardiographic images from a patient with coarctation of the aorta in the setting of hypoplasia of the aortic arch. Figure 5 (still image) and Supplementary Figure 1 (video) illustrate a three-dimensional reconstruction from a CT angiogram of a patient with coarctation of the aorta and hypoplasia of the aortic arch, in whom adequate echocardiographic imaging could not be obtained for preoperative planning. This example illustrates the challenge of echocardiographic imaging in these cases, as the air-filled trachea, which is shown in purple, can scatter ultrasound beams and limit the echocardiographic window.

Figure 4 Sagittal view of coarctation of the aorta with hypoplasia of the aortic arch ( a ) Two-dimensional sagittal image of the aortic arch. Notice the hypoplastic, long-distal transverse arch in addition to the discrete narrowing at the aortic isthmus with the posterior shelf. ( b ) Colour-compared image of the same sagittal view of the aortic arch. Notice that the colour aliasing, and therefore flow acceleration, begins in the transverse arch. LSA=left subclavian artery; PDA=patent arterial duct; BCA=brachiocephalic artery.
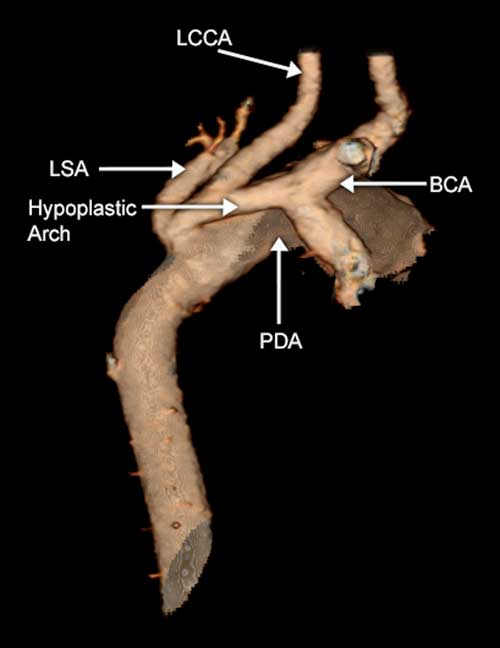
Figure 5 CT angiogram of a patient with coarctation of the aorta in the setting of hypoplasia of the aortic arch. The entire transverse arch is hypoplastic with additional discrete narrowing at the origin of the left subclavian artery (LSA). LCCA=left common carotid arteries; PDA=patent arterial duct; BCA=brachiocephalic artery.
Postoperative evaluation using echocardiography of infants and young children after neonatal repair involves monitoring for recurrent obstruction; two-dimensional measurements and Doppler interrogation of each segment of the aortic arch should be performed on each routine follow-up of these patients. Pulsed wave Doppler interrogation of each aortic arch segment helps identify the site of flow acceleration and obstruction. Monitoring trends of measurements of the aortic arch can be helpful in identifying patients who are at risk for developing re-coarctation of the aorta. Figure 6 illustrates the trend line of decreasing z-scores of the diameter of the aortic isthmus in a patient who underwent surgical repair as a neonate and developed re-coarctation of the aorta at 2 months of age.

Figure 6 Trends of z-score diameters performed via echocardiography of a patient who was initially diagnosed with a patent arterial duct (PDA) on the 1st day of life, developed severe coarctation of the aorta at the age of 2 weeks and underwent surgical repair, followed by development of re-coarctation of the aorta at 2 months of age. Measurements of the aortic isthmus are on the y-axis and time is on the x-axis. The green brackets represent 2 SD above and below the mean value for body surface area. Note that the measurement trends correspond with development of clinically significant re-coarctation of the aorta.
Imaging coarctation of the aorta in an older child
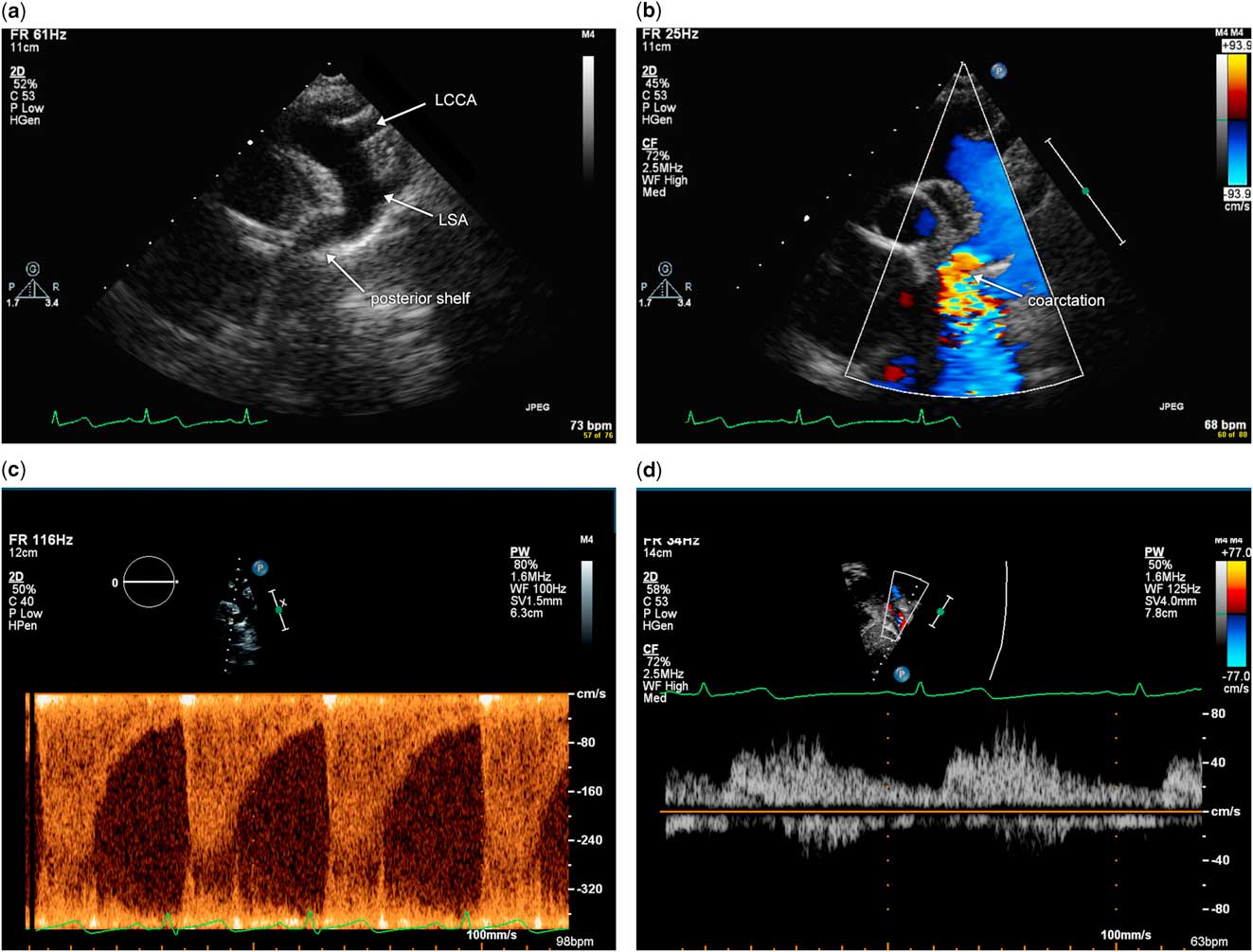
In an older child, coarctation of the aorta is typically discrete with narrowing of the aortic isthmus and absence of associated hypoplasia of the aortic arch. Imaging views via echocardiography in this population are the same as in the neonate, and include suprasternal notch views of a short-axis sweep to delineate sidedness and branching pattern of the aorta, as well as the sagittal view of the aortic arch. The diagnosis of coarctation of the aorta via echocardiography can be challenging in an older child or adolescent because of suboptimal acoustic windows and the long distance from the transducer to the aortic arch. Using low-frequency transducers and harmonic imaging may help visualise the aortic arch. Often, the high left parasternal short-axis view is needed to visualise the aortic isthmus in the older child because of the distance from the suprasternal notch. The sagittal view of the aortic arch will demonstrate narrowing and the posterior shelf at the aortic isthmus, with acceleration of flow that is evident with the use of color flow Doppler (Fig 7a and b). Pulsed wave Doppler at the isthmus demonstrates flow acceleration at the isthmus (Fig 7c). Pulsed wave Doppler of the abdominal aorta in the subxiphoid short-axis view demonstrates blunted pulsatility, consistent with severe coarctation of the aorta (Fig 7d).
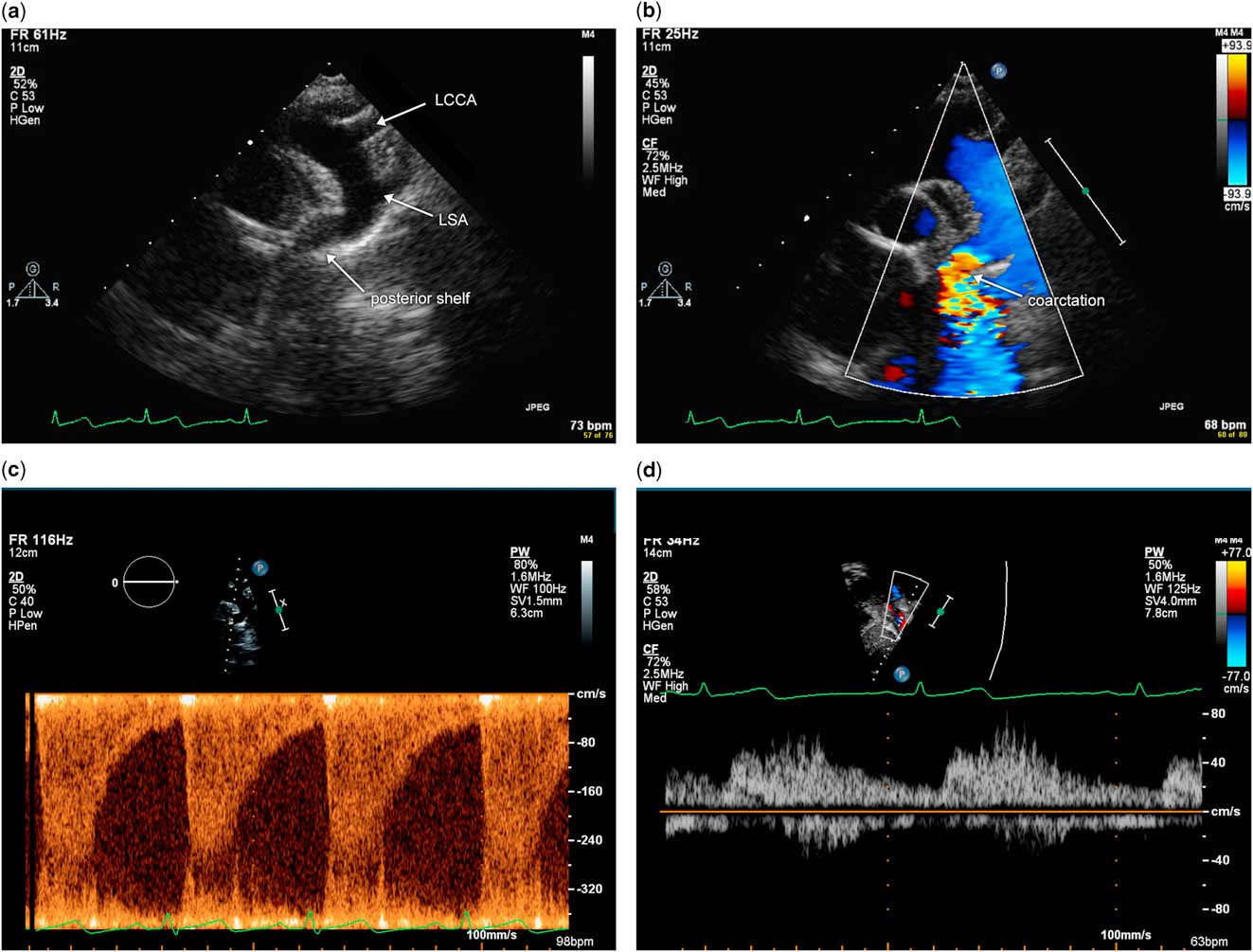
Figure 7 Illustration of echocardiographic imaging of an older child with severe coarctation of the aorta. ( a ) Sagittal view of the aortic arch from the suprasternal notch in two dimension. Note the discrete narrowing and posterior shelf at the aortic isthmus. ( b ) Sagittal view of the aortic arch from the suprasternal notch using color flow Doppler. Note colour aliasing at the aortic isthmus, which suggests acceleration of flow at this site. ( c ) Pulsed wave Doppler at the aortic isthmus demonstrates increased flow velocity at this site above 3 m/s and continuous antegrade flow in diastole. ( d ) Pulsed wave Doppler of the abdominal aorta in the subxiphoid view demonstrates blunted pulsatility with some diastolic antegrade flow. LCCA=left common carotid arteries; LSA=left subclavian artery.
Echocardiography in an older child or adolescents after repair of coarctation of the aorta can be challenging. As noted above in the younger child, it is important to screen for re-coarctation of the aorta on serial echocardiograms with Doppler interrogation of each segment of the aortic arch to identify the site and degree of obstruction; two-dimensional measurements of the aortic arch segments in the older child should ideally be performed, but the image quality can be limited in this population. In addition to development of re-coarctation, these patients can develop aneurysms at the site of repair. Therefore, periodic angiograms via CT or MRI are recommended in this population for surveillance and diagnosis. Supplementary Figures 2 and 3 illustrate an example of a patient whose aortic arch appeared normal on echocardiography (Supplementary Fig 2), but was found to have an aortic aneurysm at the site of previous surgical repair that was visualised on CT angiogram (Supplementary Fig 3).
Interrupted aortic arch
Interrupted aortic arch is defined as complete discontinuity of the aortic lumen between segments of the aortic arch. This is a separate entity from atresia of the aorta, where there is anatomic continuity of the aortic arch segments via a fibrous strand but there is obstruction of the aortic lumen, and therefore no flow between the segments. This interruption can occur at various segments of the aortic arch.
Classification
Celoria and PattonReference Celoria and Patton 10 classified interruption of the aortic arch into three types on the basis of the site of interruption in relationship to the branches of the aortic arch: types A–C. Type A is when the interruption is distal to the origin of the left subclavian artery. Type B occurs when the interruption occurs between the left carotid artery and the left subclavian arteries. Type C is when the interruption is between the right and the left common carotid arteries. In all types, the arterial duct supplies blood to the distal aorta. This classification scheme holds even in the situation of an aberrant right subclavian artery, which is when the origin of the right subclavian artery is distal to the left subclavian artery. Type B is the most common of these, and occurs in ~70% of cases, and type C is the rarest, occurring in ~1% of cases.Reference Mcrindle, Tchervenkov and Kostantinov 11
Goals of imaging
The goals of non-invasive imaging of interrupted aortic arch are described in Table 3. The objectives of imaging described are directed towards delineation of the anatomy for surgical planning. A complete echocardiogram is a part of this evaluation, and should assess for other intracardiac abnormalities that are often associated with the diagnosis of interruption of the aortic arch.
Table 3 Goals of imaging in interruption of the aortic arch.

Technical tips
Imaging views for echocardiographic assessment of interrupted aortic arch are similar to that of aortic arch obstruction as described above. The suprasternal notch is the key window for imaging, with sweeps in the short axis of the aorta and the transducer notch at 3 o'clock to determine arch-sidedness and branching as well as imaging with the transducer notch at ~12 o'clock to obtain a sagittal view of the aortic arch. If the descending aorta and proximal transverse arch/ascending aorta cannot be visualised in this view, sometimes sweeps from right to left in the suprasternal notch view can demonstrate the interruption. In addition, a high parasternal view can be utilised to visualise the descending aorta and the arterial duct separately. Key features for recognition of this diagnosis are that the ascending aorta is smaller and the main pulmonary artery is larger than normal because the ascending aorta only supplies a portion of systemic flow, whereas the pulmonary artery supplies both the pulmonary arterial flow and a significant portion of systemic flow via the arterial duct. In addition, there is complete absence of continuity between segments of the aortic arch.
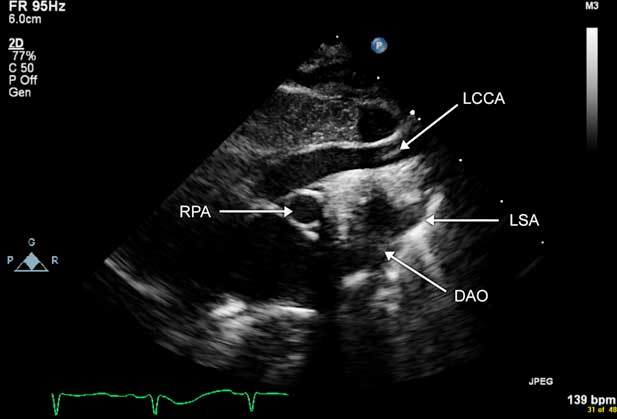
Imaging via echocardiography from the suprasternal notch can be difficult as many patients with interrupted aortic arch type B have DiGeorge syndrome, with a hypoplastic or absent thymus. The lung is often interposed between the echo transducer and the aortic arch, which makes visualisation and confirmation of the interruption and measurement of the segment of interruption difficult. Often, CT angiography or cardiac MRI is required in these patients to visualise the aortic arch more optimally for preoperative planning. Fig 8a and b illustrate imaging of interrupted aortic arch type A, and Figure 9 and Supplementary Figure 4 illustrate imaging of interrupted aortic arch type B.

Figure 8 Images of an infant with interrupted aortic arch type A with transposition of the great arteries. ( a ) Echocardiographic sagittal image from the suprasternal notch demonstrates interruption of the aortic arch distal to the left subclavian artery (LSA). ( b ) Three-dimensional reconstruction from a CT angiogram demonstrates type A interrupted aortic arch in the setting of transposition of the great arteries. This patient has a left aortic arch with normal brachiocephalic branching pattern. LCCA=left common carotid arteries; PDA=patent arterial duct; DAO=descending aorta; BCA=brachiocephalic artery; AAO=ascending aorta.

Figure 9 Echocardiographic image of an infant with type B interrupted aortic arch. This is a sagittal image of the aortic arch from the suprasternal notch view. Note the lack of continuity of the aortic arch distal to the left common carotid artery (LCCA). The left subclavian artery (LSA) is seen originating from the descending aorta (DAO). RPA=right pulmonary artery.
Long-term assessment of patients who have had repaired interrupted aortic arch is similar to that of repaired coarctation of the aorta. Evaluation of the aortic arch and intracardiac anatomy is best performed via echocardiography in younger infants and children. In older children and adolescents, CT angiography or cardiac MRI is often utilised to assess the aortic arch more optimally. Particularly in patients who have undergone primary end-to-side repair of interrupted aortic arch type B, the morphology of the repaired aortic arch may be tented superiorly. If these patients develop residual obstruction, the substrate for obstruction may be oriented more anteroposteriorly than superoinferiorly. This can result in suboptimal angulation for interrogation by Doppler, resulting in the under-diagnosis, or missed diagnosis, of obstruction. If this is the case, supportive evidence such as blunting of the pulsatility of flow in the abdominal aorta or the development of new or increased left ventricular hypertrophy and/or dysfunction may warrant cardiac catheterisation.
Late complications following repair of interruption of the aortic arch include recurrent obstruction of the aortic arch and aortic aneurysms at the site of repair. Left ventricular outflow tract obstruction can be noted in the postoperative period. Evaluation of size and function of the left ventricle is also an important part of long-term monitoring of this population, as progressive hypertrophy and dysfunction of the left ventricle can provide evidence of significant obstruction.
Conclusions and future directions
Echocardiography is the most frequently used modality for the recognition of abnormalities of the aortic arch in children; however, because of airway and lung tissues obscuring the echocardiographic windows of these patients, sometimes the entire aortic arch anatomy cannot be visualised adequately for surgical planning. In addition, in older patients, late aortic aneurysms after surgical or interventional approaches are challenging to recognise via echocardiography. CT or MRI angiography can be useful for optimal imaging in these cases, and is being utilised more frequently as the technology has improved and is becoming more accessible.
Acknowledgements
None.
Financial Support
The images used for this article were obtained from clinical images without the use of grant funding or other financial support.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1047951116001670