Mitral valve diseases are common at all ages,Reference Falk, Baumgartner and Bax1–Reference Lancellotti, Moura and Pierard3 including children.Reference Cantinotti, Scalese, Molinaro, Murzi and Passino4–Reference Bairacharya, Bhatnagar and Pauliks42 In the paediatric age group, mitral valve diseases may be congenital or acquired and may present either in isolation or within the context of other congenital heart lesions. Significant anomalies of the mitral valve account for 0.4% of CHDs; however, minor anomalies of the mitral valve may be detected in up to 4% of school-aged children.Reference Remenyi and Gentles8–Reference Abdullah, Pearce, Palmer and Chenzbraun19,Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Rallidis, Voltyrakis, Vrettou, Filippatos, Paraskevaidis and Kremastinos50 Echocardiographic evaluation of mitral valve diseases in the paediatric age group requires a careful sequential analysis of multiple valvular elements (annulus, leaflets, chordae, inter-chordal space, and papillary muscles) and knowledge of anatomical variants and of associated CHD. Beside the anatomical analysis, a quantitative and semi-quantitative evaluation of disease severity (i.e. the grade of stenosis and/or regurgitation) is also required. Guidelines for the management of valvular heart disease in adults have recently been revised,Reference Falk, Baumgartner and Bax1–Reference Lancellotti, Moura and Pierard3 but their utility in children (from 0 to 18 years of age) has been poorly evaluated. Currently, in children, there is no standardised approach for the echocardiographic quantitative and or semi-quantitative echocardiographic assessment of mitral valve disease and no validated classification of their severity of stenosis or regurgitation in children exists. Parameters and cut-off values used in adults have been applied to childrenReference Cantinotti, Scalese, Molinaro, Murzi and Passino4–Reference Lopez, Colan and Frommelt6 without any validation. There are many factors in the child with CHD which complicate reliable quantitative assessment of atrio-ventricular valve stenosis or regurgitation. These include (1) the massive range of body size negating the use of “cut off values” with respect to, for example, valve area or effective regurgitant orifice area; (2) the diversity of morphology even within a single valve category which may impact the shape and location or regurgitant zones; (3) changes in myocardial function with age which may impact on Doppler – derived gradients across valves; (4) the impact of changes of heart rate with age/growth which may lead, for example, to fusion and summation of inflow velocities; (5) indexation of any values such as valve area or effective regurgitant orifice area for body size (e.g. how to do?); (6) impact of intra-cardiac shunts on volume flow or flow redistribution within/outside the heart.
Over the past decade, the introduction and refinement of three-dimensional echocardiographic techniques means that real-time techniques can be applied to great en face views of the mitral valve to measure parameters such as annulus size and shape, valvar area, and the size and shape of regurgitant jets.Reference Simpson, Lopez and Acar46
The aim of the present work is to review the current literature on paediatric mitral valve disease, focusing on the heterogeneity, limitations of current echocardiographic classification systems, and the lack of robust quantitative or semi-quantitative criteria for mitral stenosis and regurgitation quantification. The potential for new three-dimensional techniques will be also addressed.
Methods
Search strategy
Potential publications were identified from a systematic search in the National Library of Medicine (PubMed access to MEDLINE citations, http://www.ncbi.nlm.nih.gov/PubMed/) conducted in April 2018. The search strategy included a mix of MeSH and free text terms for the key concepts starting from mitral, stenosis, and regurgitation in children. The search was further refined by adding the keywords echocardiographic definition, classification, or evaluation. In addition, we identified other potentially relevant publications using a manual search of references from all eligible studies and review articles as well as from Science Citation Index Expanded on Web of Science. Titles and abstracts of all articles identified by this search strategy were evaluated, and manuscripts were excluded if (a) the study population involved mixed adult and paediatric populations; (b) used definitions or classification systems that were not based on echocardiographic parameters; (c) written in a language different from English; (d) involved neonatal critical diseases, as these lesions represent a different category of valvular heart disease; and (e) the study involved mitral valve (left atrio-ventricular valve) in the univentricular heart. Finally, analysis of rheumatic mitral valve disease was excluded as it was beyond the scope of this search. The review focuses on articles reporting quantitative and semi-quantitative methods for mitral valve stenosis and regurgitation quantification. All articles were assessed independently by two reviewers and included into the study after consensus was reached.
Results
Search results
Out of 75 publications identified for potential inclusion into the study, 44 (58.6%) studies were excluded based on the criteria listed above, while 31 (41.4%) were finally selected for analysis and the following systematic review.
Methodological limitations
Limitations in methodology were seen in most of the studies. Several reports presented data on only a limited number of healthy control subjects, and some described findings in children from infants to teenagers with total sample sizes less than 50 subjects.Reference Aotsuka, Tobita and Hamada28,Reference Baspinar, Karaaslan and Oran29,Reference Ziani, Lactu, Abadir, Paranon, Dulac, Guerrero and Acar31 This makes it impossible to create size or age specific normal ranges. Because of the absence of a standardised methodology based on expert consensus to measure valvular gradients and severity of regurgitation in the paediatric population, all studies used different approaches. Systems to classify mitral stenosis severity have been rarely reported, and when employedReference Banerjee, Kohl and Silverman10, Reference Collins-Nakai, Rosenthal, Castaneda, Bernhard and Nadas12 different cut-off values have been used to define mild, moderate, and severe stenosis. For regurgitant lesions, variable echocardiographic views (four-chamber and/or parasternal views)Reference Baspinar, Karaaslan and Oran29–Reference Ziani, Lactu, Abadir, Paranon, Dulac, Guerrero and Acar31 and parameters to evaluate disease severity (i.e. proximal isovelocity surface area or vena contracta) have been used. In addition, few studies addressed technical issuesReference Bonow, Carabello and Kanu2,Reference Lancellotti, Moura and Pierard3,Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43,Reference Ricek44 related to settings on the echocardiographic systems (such as image and colour gain as well as the Nyquist limit for Doppler interrogation).
General principles
For stenotic lesions, the “ideal” echocardiographic evaluation should provide the following information: (1) valvular anatomy; (2) quantitative parameters such as trans-valvular pressure gradient, orifice area, and annular diameter; (3) semi-quantitative assessment of the left ventricle, right ventricle, left atrium, and right atrium (i.e. diameters, volumes, left ventricle wall thickness and/or mass, and systolic and diastolic function; and (4) associated CHDs. Similarly, evaluation of regurgitant lesions should include both qualitative and quantitative assessment of the regurgitant jet.
The diagnosis of mitral valve defects is generally made by trans-thoracic two-dimensional echocardiography.Reference Falk, Baumgartner and Bax1–Reference Cantinotti, Scalese, Molinaro, Murzi and Passino4 Other modalities including three-dimensionalReference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Valverde, Rawlins, Austin and Simpson47 and trans-oesophageal echocardiography may provide additional information to guide management.Reference Remenyi and Gentles8, Reference Seguela, Houyel and Acar9
Anatomical evaluation
A detailed anatomical description of the stenotic and/or insufficient mitral valve anatomy and its apparatus is required through a segmental approach of the supra-valvular region, annulus, leaflets, commissures, chords, and papillary muscles.Reference Remenyi and Gentles8, Reference Seguela, Houyel and Acar9, Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Baird, Marx, Borisuk, Emani and del Nido48 In mitral valve defects in fact usually all the components of the mitral valve apparatus (e.g. supra-valvular, annular, and sub-valvular region) may take part in the lesion.Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9,Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Baird, Marx, Borisuk, Emani and del Nido48 The supra-valvular region should be explored in order to detect the presence of a mitral ring, an anomaly that usually present in association with left-sided obstructive lesions.Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9,Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Simpson, Lopez and Acar46 The size of the annulus (that may be hypoplasic or dilated) needs to be carefully evaluated and compared with range of normality according to paediatric nomograms.Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9,Reference Anderson15 Leaflets may present various anomalies including thickness, dysplasia, deficiency, underdevelopment, cleft, motion abnormalities (restriction or prolapse regarding one or more scallops or the entire area), and presence of accessory tissue.Reference Remenyi and Gentles8, Reference Seguela, Houyel and Acar9,Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Baird, Marx, Borisuk, Emani and del Nido48 The commissures furthermore may be underdeveloped or absent.Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9,Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Baird, Marx, Borisuk, Emani and del Nido48 Attachment and anatomical detail oftendinous chordae (thickening, shortening versus elongation, intracordal spaces obliteration/fusion) also need to be evaluated.Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9,Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Baird, Marx, Borisuk, Emani and del Nido48 Finally, the detailed papillary muscles anatomy should be assessed for their number, size, and location, regarding hypertrophy/hypoplasia, agenesis, fusion, elongation, and position/symmetry (e.g. location higher in the left ventricle) (Tables 1 and 2).Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9,Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Baird, Marx, Borisuk, Emani and del Nido48
Table 1. Congenital MV disease: major classification systems proposed in literature

HLHS = hypoplastic left heart syndrome; MR = mitral regurgitation; MS = mitral stenosis; MV = mitral valve.
Table 2. Congenital mitral valve disease: definition, anatomical and echocardiographic detail, and associated anomalies. Authors’ summary
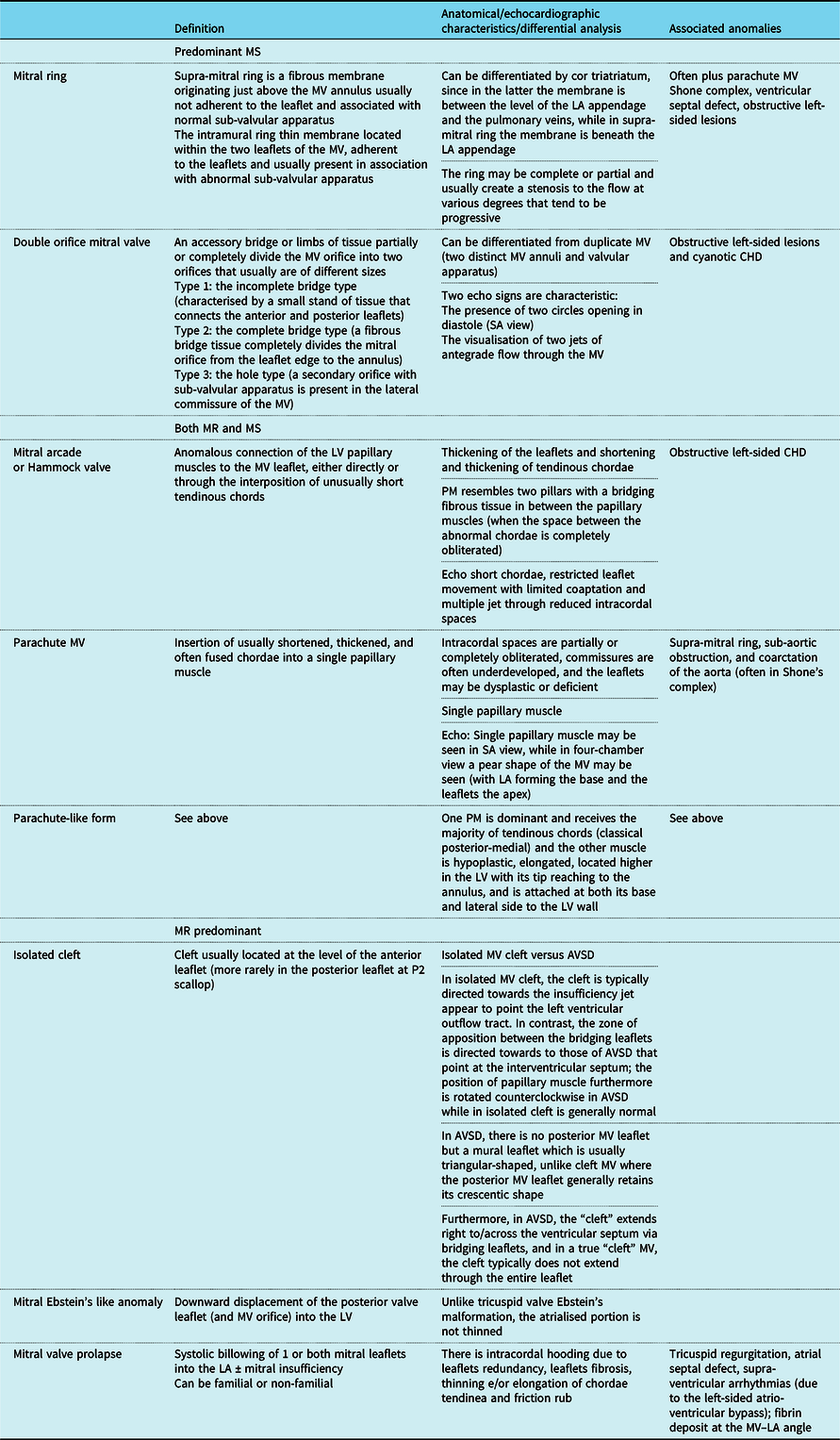
AVSD = atrio-ventricular septal defect; MR = mitral regurgitation; MS = mitral stenosis; MV = mitral valve; LA = left atrium; LV = left ventricle; SA = short axis.
Specific lesions
Congenital mitral valve defects
Various anatomical and functional classification systems have been used to describe congenital mitral valve defectsReference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9,Reference Carpentier, Branchini and Cour11–Reference Oppido, Davies and McMullan14 according to the predominant lesion,Reference Carpentier, Branchini and Cour11 associated lesions,Reference Collins-Nakai, Rosenthal, Castaneda, Bernhard and Nadas12 segmental approach,Reference Mitruka and Lamberti13,Reference Oppido, Davies and McMullan14 hemodynamic evaluation (predominant mitral regurgitation or stenosis),Reference Mitruka and Lamberti13,Reference Oppido, Davies and McMullan14 functional analysis (leaflet prolapse/restricted leaflet motion),Reference Oppido, Davies and McMullan14 and presence/absence of leaflet dysplasia.Reference Oppido, Davies and McMullan14 The presence of “non-classic” forms of mitral valve disease that are frequently encountered and the overlapping of some lesions that classically have been considered as distinct entity may at least in part explain these discrepancies in the classification systems.Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9,Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Baird, Marx, Borisuk, Emani and del Nido48 In Table 1, an overview of major classification systems published so far for congenital mitral stenosis has been reported, while in Table 2 a summary of the definitions and anatomical substrates of various congenital mitral defects has been provided (Table 2).
Mitral stenosis
Mitral valve stenosis rarely occurs as an isolated congenital defect. It presents more commonly as an acquired lesion, or in association with multi-level left heart hypoplasia or other CHDs.Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9,Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Valverde, Rawlins, Austin and Simpson47 Most complex congenital mitral defects including double orifice mitral valve,Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9,Reference Ricek44–Reference Simpson, Lopez and Acar46 supra-mitral ring,Reference Baird, Marx, Borisuk, Emani and del Nido48 mitral arcade,Reference Seguela, Houyel and Acar9,Reference Ricek44 and parachute mitral valveReference Seguela, Houyel and Acar9,Reference Ricek44 are typically associated with mitral valve stenosis.
Quantitative evaluation
In adults, cut-off values for various parameters are used to define mild, moderate, and severe mitral stenosis including the mean trans-valvular gradient (<5, 5–10, >10 mmHg), pulmonary artery systolic pressure (<30, 30–50, >50 mmHg), and valve area (>1.5, 1.0–1.5, <1.0 cm2).Reference Bonow, Carabello and Kanu2 No parameters for the echocardiographic quantification of mitral stenosis severity have been fully validated for a paediatric population,Reference Lopez, Colan and Frommelt6 and only a few paediatric works proposed mitral valve stenosis classifications according to gradients derived either by pulsed DopplerReference Banerjee, Kohl and Silverman10 or cardiac catheterisation.Reference Collins-Nakai, Rosenthal, Castaneda, Bernhard and Nadas12 The range of gradients proposed to define mild, moderate, and severe mitral stenosis, however, is very discordant by publicationReference Banerjee, Kohl and Silverman10,Reference Collins-Nakai, Rosenthal, Castaneda, Bernhard and Nadas12 and differs from adult recommendations (Table 3).Reference Lopez, Colan and Frommelt6 In children, quantitative estimation of mitral valve stenosis includes at least the evaluation of trans-valvular pressure gradient, orifice area, and annular diameter.Reference Lopez, Colan and Frommelt6 All these parameters, however, are affected by significant physiologic variations with growth, and thus cut-off values to estimate disease severity (if applicable) should be modulated according to age and body size. The impact of other co-existent shunts (e.g. ventricular septal defects which may increase trans-mitral flow, or atrial septal defects which may reduce mitral valve flow by permitting shunting to the right atrium) also needs to be considered. The Doppler velocity time integral of the inflow tracing from mitral valve inflow velocity waves is commonly employed to estimate the mean gradient. However, studies comparing the accuracy of gradient estimated at echo with those derived from invasive assessment are limited.Reference Lopez, Colan and Frommelt6,Reference Banerjee, Kohl and Silverman10 Banerjee and colleagues,Reference Banerjee, Kohl and Silverman10 in 14 children (0–18 years) with congenital mitral valve stenosis of various nature, demonstrated fair correlation (r = 0.75) between mean mitral valve pressure gradient obtained by Doppler and cardiac catheterisation. In contrast,Reference Banerjee, Kohl and Silverman10 the correlation between the mitral valve areas calculated by the Doppler pressure half-time method and by the Gorlin formula was poor (r = 0.57).
Table 3. Cut-off employed to define mitral stenosis severity in different studies.
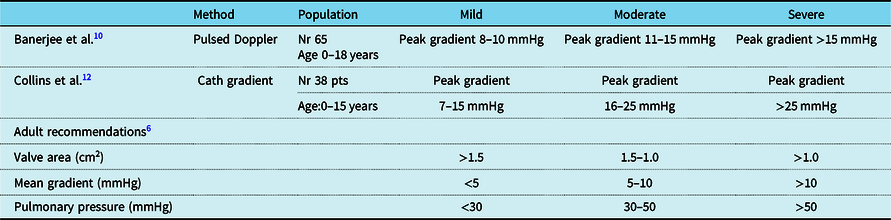
Theoretically, velocity time integral should be evaluated with reference to normative data, considering that mitral valve velocity waves profiles change with age.Reference Cantinotti, Giordano and Scalese51 The peak blood flow early diastolic velocity (E wave) increases up to 5 years of age and stabilises thereafter (with little age variations),Reference Cantinotti, Giordano and Scalese51 while the peak blood flow in late diastolic velocity (A wave) increases within the first 12 months but decreases thereafter. Paediatric nomograms for mitral valve velocity time integral, however, are absent. Furthermore, mitral valve inflow flow velocities are dependent on the diastolic filling period and can be augmented by faster heart rates in infants and children (particularly in the neonatal age)Reference Ricek44 that remain a major issue.Reference Lopez, Colan and Frommelt6,Reference Banerjee, Kohl and Silverman10 Recently, z scores for prosthetic mitral valve have been proposedReference Hayashi, Inuzuka, Ono and Kato52 for various indexes including peak E velocity (m/s), mean pressure gradient, velocity time integral ratio of prosthetic mitral valve inflow to left ventricular out flow body surface area-indexed effective orifice area (cm2/m2), and pressure half time. However, these z scores have been built by using a very small sample size (e.g. 24 examinations performed at various times in 12 children (age 6.6–18.1 years)).Reference Hayashi, Inuzuka, Ono and Kato52
Measurements of the annulus are quite reproducible by two-dimensional echocardiography and robust nomograms are available.Reference Cantinotti, Kutty and Franchi53 In contrast, mitral valve area can be assessed by two-dimensional echocardiography; however, validation with invasive catheterisation measurements has shown contrasting resultsReference Banerjee, Kohl and Silverman10,Reference Riggs, Lapin, Paul, Muster and Berry24 and nomograms are limited.Reference Cantinotti, Scalese, Molinaro, Murzi and Passino4 Furthermore, congenital mitral stenosis often includes multi-level obstruction and thus the narrowest point may be above the annulus (e.g. supra-mitral l ring) or at a lower level (funnel-shaped mitral valve). In this context, the use of 3D echo may help for a better visualisation of the whole mitral valve anatomy and a more accurate estimation of the real orifice at the narrowest point.Reference Simpson, Lopez and Acar46,Reference Rallidis, Voltyrakis, Vrettou, Filippatos, Paraskevaidis and Kremastinos50 However, nomograms for mitral valve evaluated by 3D echocardiography are limited.Reference Cantinotti, Kutty and Franchi53
Evaluation of left heart parameters and indirect estimation of pulmonary pressure
Evaluation of left atrium and left ventricle dimensions and function is an essential part of estimation of mitral stenosis severity.Reference Bonow, Carabello and Kanu2,Reference Lancellotti, Moura and Pierard3 In moderate-to-severe mitral valve disease, the left atrium wall bulges to the right, and the left atrium is usually dilated.Reference Bonow, Carabello and Kanu2,Reference Lancellotti, Moura and Pierard3 The left ventricle may be dilated when there is both mitral valve stenosis and regurgitation or may be hypoplastic in patients with Shone’s complex.Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Baird, Marx, Borisuk, Emani and del Nido48 When evaluating left atrium dilatation, it is important to index measurements. z scores for the paediatric population have been recently become available.Reference Cantinotti, Scalese, Molinaro, Murzi and Passino4 The presence of tricuspid insufficiency should be monitored for indirect quantification of pulmonary pressure and response to exercise may be important.Reference Lopez, Colan and Frommelt6 In the neonatal period, the presence of a patent foramen ovale needs to be carefully evaluated for quantification of differences among atrial pressure and to estimate the degree of left to right shunt that may an indirect indicator of inflow obstruction and left ventricle non-compliance.Reference Hasan, Parvez and Ajmal54 The potential to shunt at atrial level also confounds measurements of trans-mitral flow.
Mitral valve regurgitation
Acute mitral regurgitation is extremely rare in children and may be due to trauma,Reference Falk, Baumgartner and Bax1,Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9 idiopathic (due to acute chordal rupture),Reference Okada, Inoue and Fukushima55 or more often iatrogenic following surgical or catheter intervention.Reference Falk, Baumgartner and Bax1,Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9 Chronic mitral regurgitation instead is common and may present in isolationReference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9 or more commonly in the context of other cardiac/systemic disease. Secondary causes of mitral regurgitation include connective tissue diseases (i.e. Marfan syndrome), rheumatic disease (especially in developing countries), and various CHDs (before and after correction/palliation).Reference Lopez, Colan and Frommelt6,Reference Bonow, Cheitlin, Crawford and Douglas23–Reference Hlavacek Am, Chessa and Shirali33 Mitral regurgitation may be found in association with stenosis in all complex congenital mitral defects, as detailed in Tables 1 and 2. Rare anatomical defects of mitral valve apparatus that may cause mitral regurgitation include isolated cleft of the mitral valve,Reference Seguela, Houyel and Acar9,Reference Tamura, Manahem and Bizard34,Reference Ricek44,Reference Séguéla, Dulac and Acar45 mitral arcade or hammock valve,Reference Remenyi and Gentles8,Reference Seguela, Houyel and Acar9,Reference Acar, Laskari, Rhodes, Pandian, Warner and Marx32,Reference Ricek44,Reference Séguéla, Dulac and Acar45 and Ebstein’s like anomaly (Tables 1 and 2).Reference Ruschlhaupt, Bharati and Lev35
Quantitative and semi-quantitative assessment
A quantitative assessment is often important to guide management of children with mitral regurgitation.Reference Bonow, Cheitlin, Crawford and Douglas23–Reference Hlavacek Am, Chessa and Shirali33 At the present time, there are no clear criteria to grade mitral regurgitation in children. New echocardiographic indices and classification systems for semi-quantitative and quantitative evaluation have been proposed in adults; however, their validity and reproducibility in children are unknown.Reference Cantinotti and Lopez5 The use of vena contracta that is strongly advised in adultsReference Falk, Baumgartner and Bax1–Reference Lancellotti, Moura and Pierard3 was validated in a single paediatric studyReference Prakash, Lacro and Sleeper25 (Table 4). This study evaluated mitral regurgitation in 149 infants after biventricular correction for atrio-ventricular septal defect. The lateral, antero-posterior, and cross-sectional vena contracta area indexed for body surface area correlated moderately with z scores of left ventricular end diastolic volumes and showed high inter-observer agreement.Reference Prakash, Lacro and Sleeper25 The use of flow convergence method is now the most recommended approach for a quantitative evaluation of mitral regurgitation in adults, but this has been tested only in a few paediatric studies.Reference Aotsuka, Tobita and Hamada28,Reference Baspinar, Karaaslan and Oran29 These studies were all numerical limited included (less than 35 patients) and evaluated a wide range of patient ages from the neonatal period to adult life.Reference Aotsuka, Tobita and Hamada28,Reference Baspinar, Karaaslan and Oran29 Technical issues including application of adult Nyquist limits (15–40 cm/s) at the high heart rates encountered in the paediatric age have not been addressed. The use of three-dimensional echocardiography may provide additional information in the evaluation of mitral regurgitation by the proximal isovelocity surface area method.Reference Gabriel Lactu, Paranon and Bongard30 A small study by Ziani and colleagues (31 patients 1 month–20 years) showed the benefit of three-dimensional echocardiography to describe the true proximal isovolumetric surface area shape in children with mitral regurgitation. They showed that the proximal isovelocity surface area shape was more often prolate or oblate hemispheroidReference Ziani, Lactu, Abadir, Paranon, Dulac, Guerrero and Acar31 than hemispherical (Tables 4 and 5). Significant differences were observed among regurgitant volume calculated by two-dimensional and three-dimensional methods. In contrast, in a study of 54 children from 1 to 19 years with mitral regurgitation,Reference Gabriel Lactu, Paranon and Bongard30 proximal isovelocity surface area by 3D echocardiography was well correlated with regurgitant volume (r = 0.83) and regurgitant fractions (r = 0.79). Another small paediatric studyReference Baspinar, Karaaslan and Oran29 showed good correlation of effective regurgitant area and regurgitant volume by the proximal isovelocity surface area method with those obtained by Doppler. The presence of multiple regurgitant jets in the setting of repaired atrio-ventricular septal defect is another issue to be considered (Figs 1–3 and Supplementary videos 1 and 2).Reference Calkoen, Westenberg and Kroft56,57 A multimodal approach combining echocardiography and MRI may enable improved evaluation of ventricular volumes and flows and quantification of multiple regurgitant jets after atrio-ventricular septal defect repair.Reference Calkoen, Westenberg and Kroft56 A study of 32 cases (age 26 ± 12 years) with mitral regurgitation after atrio-ventricular septal defect correction reported poor correlations between qualitative echocardiographic grading and MRI methods (r = 0.51).Reference Calkoen, Westenberg and Kroft56 Studies comparing quantitative echocardiographic assessment of mitral regurgitation with MRI are lacking.
Table 4. Adult recommendations to quantify mitral regurgitation severity
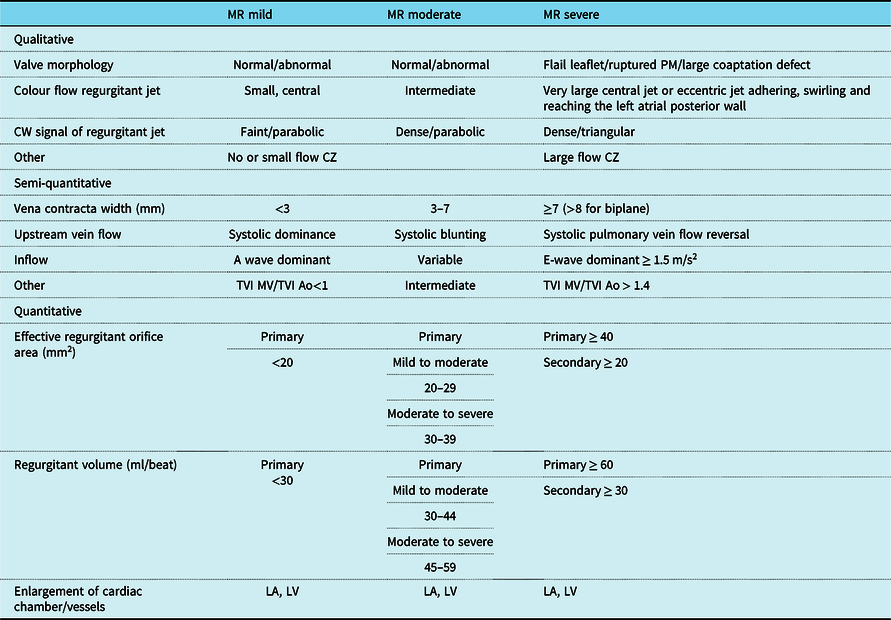
Ao = aorta; CZ = convergence zone; LA = left atrium; LV = left ventricle; MR = mitral regurgitation; MV = mitral valve; TVI = time velocity integral.
Table 5. A few studies evaluating diagnostic accuracy of some echocardiographic parameters to grade mitral regurgitation in children

Ao = aortic; 2D = two dimensional; AVSD = atrio-ventricular septal defect; BSA = body surface area; EROA = effective regurgitant orifice area; ICC = intraclass correlation coefficient; i-VCW ap = vena contracta width anterior-posterior indexed by BSA; LVEDV = left ventricular end diastolic volume; MR = mitral regurgitation; MV = mitral valve, MVP = mitral valve prolapse, PISA = proximal isovelocity surface area, r = PISA radius; RF = regurgitant fraction; RJA = regurgitant jet area (measured by planimetry from the frame with the maximal systolic regurgitant jet area); RV = regurgitant volume, r is radius of proximal flow convergence and Vr is aliasing velocity; V = aliasing velocity; VC = vena contracta.
* Subjects with >1 jet of regurgitation were excluded. BSA formula was not specified.
** The jet length was defined as the maximal distance of the regurgitant signals from the MV orifice.
*** EROA = (2r 2) × (the velocity at which the aliasing surface occurred/peak velocity of the MR and VS jet).
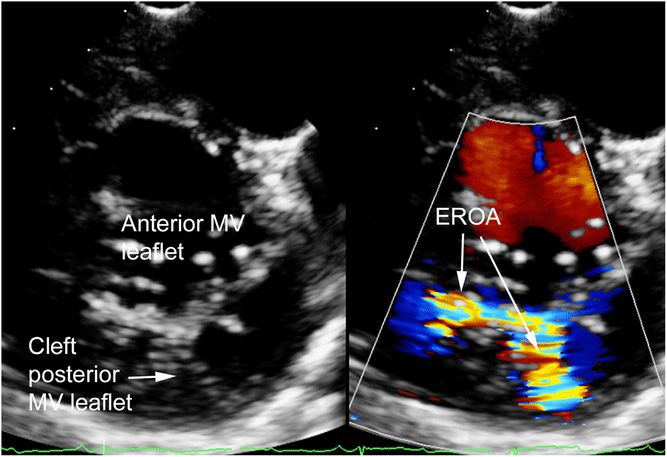
Figure 1. 2D short-axis MV view showing regurgitation with isolated posterior MV cleft. EROA = effective regurgitant orifice area; MV = mitral valve. See Table 3 for definition of isolated MV cleft.
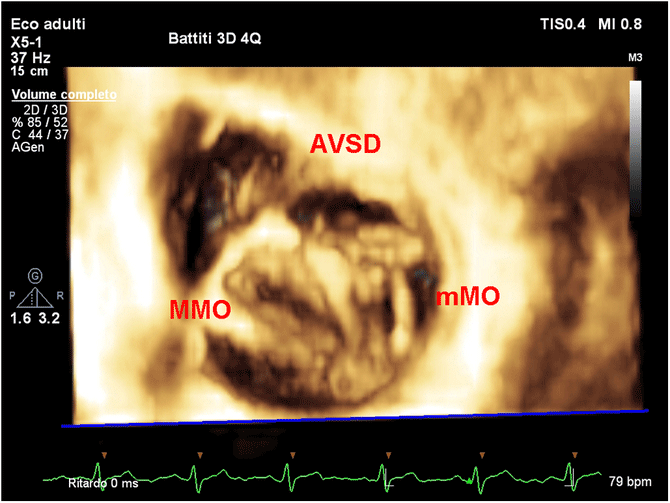
Figure 2. 3D en face view showing a double orifice mitral valve in AVSD. AVSD = atrio-ventricular septal defect; MMO = major mitral orifice; mMO = minor mitral orifice.
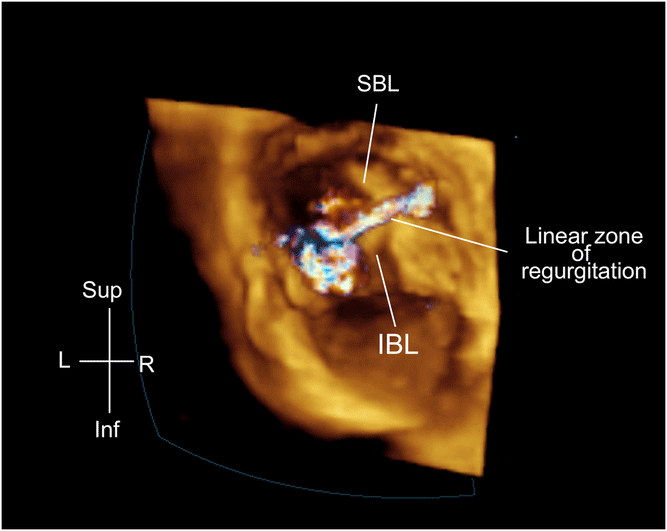
Figure 3. 3D en face view showing a linear left atrial valve regurgitation post-AVSD repair. AVSD = atrio-ventricular septal defect; SBL = superior bridging leaflet; IBL = inferior bridging leaflet.
Evaluation of left heart parameters and indirect estimation of pulmonary pressure
Analogous consideration made for mitral stenosis is also valid for mitral regurgitation, where the volumes and function of the left heart and evaluation of pulmonary pressures play an essential role in the estimation of disease severity.Reference Bonow, Carabello and Kanu2,Reference Lancellotti, Moura and Pierard3 Pulmonary vein systolic reversal is another important indicator of severe mitral regurgitation.Reference Bonow, Carabello and Kanu2,Reference Lancellotti, Moura and Pierard3
Newer techniques: three-dimensional echocardiography
Multiple case reportsReference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43,Reference Séguéla, Dulac and Acar45–Reference Baird, Marx, Borisuk, Emani and del Nido48 and review papersReference Ricek44 show that the use of 3D echocardiographyReference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Baird, Marx, Borisuk, Emani and del Nido48 may help to better appreciate the anatomy of complex mitral stenosis including double orifice mitral valve,Reference Séguéla, Dulac and Acar45–Reference Jolley, Hammer and Ghelani49 mitral ring,Reference Baird, Marx, Borisuk, Emani and del Nido48 mitral archade,Reference Ricek44 and parachute mitral valveReference Seguela, Houyel and Acar9,Reference Ricek44 (Fig 2 and Supplementary video 1). Trans-thoracic three-dimensional echocardiographic evaluation of the mitral valve is made by using multiple views including parasternal long-axis, parasternal short-axis view, and apical four chamber.Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Baird, Marx, Borisuk, Emani and del Nido48 Trans-oesophageal projections include four-chamber view at a mid-oesophageal level at 0–30°, a mid-oesophageal level at 120–130°, or a trans-gastric view at 0–30° (i.e. short-axis view).Reference Miglioranza, Muraru, Mihaila, Haertel, Iliceto and Badano43–Reference Baird, Marx, Borisuk, Emani and del Nido48 In mitral stenosis, the use of 3D echocardiography may help to plan the surgical strategy, by en face view of valve anatomy and the identification of the principal site of stenosis (valvular, supra-valvular, or sub-valvular).Reference Simpson, Lopez and Acar46 In addition, the anatomy of chordal support apparatus can be defined in a manner that is not possible by two-dimensional echocardiography. Similarly for interventional purpose in regurgitant lesions, 3D echocardiography may allow to better identify the presence of cleft, leaflets prolapse (Fig 3 and Supplementary video 2), and the degree size, shape, number, and location of multiple regurgitant jets in repaired atrio-ventricular septal defect lesions such as atrio-ventricular septal defect.Reference Calkoen, Westenberg and Kroft56,57 Furthermore, three-dimensional echo may allow for a more precise measurement of the mitral valve annulus estimation of the real mitral annulus (that can be misinterpreted with single plane views),Reference Séguéla, Dulac and Acar45–Reference Valverde, Rawlins, Austin and Simpson47,Reference Rallidis, Voltyrakis, Vrettou, Filippatos, Paraskevaidis and Kremastinos50 mitral valve area, effective regurgitant area, and proximal isovelocity surface area calculationReference Gabriel Lactu, Paranon and Bongard30,Reference Ziani, Lactu, Abadir, Paranon, Dulac, Guerrero and Acar31 (Supplementary video 3).
Concluding remarks
There are multiple and heterogeneous approaches to the evaluation and management of paediatric atrio-ventricular valves disease. Adult guidelines cannot be simply translated to children. Consensus documents, based on current studies and expert consensus (when evidences are lacking), are needed to identify the limitations in the current classification systems and to establish practice standards in terms of treatment and follow-up. Recommendations should be based on anatomic and functional findings. In addition, paediatric recommendations must account for the effects of age, particularly in terms of the faster heart rates, and poor patient cooperation in neonates and infants.Reference Hayashi, Inuzuka, Ono and Kato52 These issues will have technical implications, including the settings used during the echocardiographic examination. Any evaluation must also address the presence of associated defects as they may influence management approaches. The role of an integrated multi-imaging approach with MRI or cardiac CTReference Calkoen, Westenberg and Kroft56 should also be discussed.
A consensus document57 could highlight the gaps of evidence and propose new approaches to overcome them. New studies should be based on wider populationReference Williams, Thomson and Seto58 and evaluate multiple indices including pressure gradients, vena contracta, proximal isovelocity surface area, effective regurgitant area, regurgitant fraction, and chamber dimensions. They should also integrate newer indices derived from three-dimensional echocardiography and strain analysis and provide comparisons with MRI and invasive data in specific clinical situations.
Acknowledgements
None
Financial Support
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Employment or Leadership
None declared.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951119003196










