A 27-year-old previously healthy woman has two children aged 1 and 2 years, and had no complications with pregnancies or deliveries. She developed symptoms of dyspnea, fatigue, lower extremity oedema, abdominal distention, bloating, and a 30-pound weight gain over a 1-month period. She was first treated for gastroenteritis. A subsequent cardiology evaluation showed giant P waves on electrocardiogram. Transthoracic echocardiography revealed a markedly dilated right atrium, marked displacement of the tricuspid valve orifice up towards the right ventricular outflow tract, a diminutive functional right ventricle and suspicion for a right ventricular outflow tract obstruction/compromise due to thrombus with mean gradient of 4–5 millimetres of mercury. In addition, left ventricular size was reduced from leftward displacement of the ventricular septum; left ventricular function was normal. Cardiac magnetic resonance imaging was performed to better assess right ventricular function and anatomy of the tricuspid valve and right ventricular outflow tract. Magnetic resonance imaging showed tricuspid valve leaflet tissue in the right ventricular outflow tract adjacent to the pulmonary valve with organised thrombus in the small functional right ventricle between the right ventricular outflow tract and the right ventricular apex. Pre-operative imaging was previously published.Reference Tabatabaei, Katanyuwong and Breen1 The diagnosis of severe Ebstein’s malformation or one of its variants such as an unguarded tricuspid valve orifice was made. The patient was started on diuresis and anticoagulation. The formation of the clot was mostly due to low cardiac output, and specifically low right-sided output with very sluggish flow noted in the right cardiac chambers. In addition, a hypercoagulable workup was performed and was positive for lupus anticoagulant factor and the patient was started on lifelong anticoagulation. The functional tricuspid stenosis and low right-sided cardiac output resulted in persistent class IV cardiac failure despite medical therapy and she was referred for operation.
Operation consisted of right ventricular thrombectomy, right atrial reduction, porcine tricuspid valve replacement, positioned at a level just cephalad to the true annulus, that is, atrioventricular groove, and bidirectional cavopulmonary anastomosis, that is, “bidirectional Glenn”. At operation, we documented the presence of displaced tricuspid leaflet tissue in the right ventricular outflow tract confirming the diagnosis of Ebstein malformation. Her post-operative course was uneventful.
She has been stable clinically more than 1 year after operation. Pre- and post-operative echocardiographic images are shown (Figs 1–4). Magnetic resonance images are also shown (Figs 5 and 6); pre- and post-operative right ventricular end diastolic volume indexed to body surface area was 40 and 28 millilitres per square metres, respectively. Pre- and post-operative right ventricular ejection fraction was 24 and 54%, respectively.
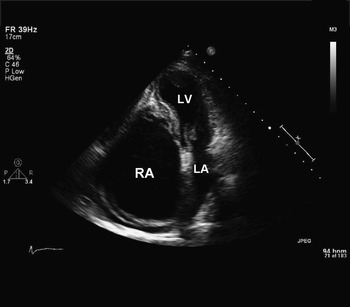
Figure 1 Two dimensional transthoracic echocardiogram – apical four-chamber view – before surgery, revealing a very dilated right atrium, diminutive right ventricle; RA, right atrium; LA, left atrium; LV, left ventricle.

Figure 2 A Two-dimensional transthoracic echocardiogram – (a) parasternal long axis view and (b) parasternal short axis view – before surgery; raising the suspicion for a right ventricular outflow tract obstruction/compromise due to thrombus with mean gradient of 4–5 millimetres of mercury (asterisk); LV, left ventricle; RVOT, right ventricular outflow tract.

Figure 3 A two-dimensional transthoracic echocardiogram – parasternal long axis view – before surgery; RV, right ventricle; LA, left atrium; LV, left ventricle; Ao, aorta.

Figure 4 A two-dimensional transthoracic echocardiogram – apical four-chamber view – after surgery, showing bioprosthetic tricuspid valve (arrow); RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle.

Figure 5 (a) Pre-operative and (b) post-operative magnetic resonance images – oblique sagittal view of the right atrium and right ventricle passing through the atrioventricular groove. Contrast-enhanced blood pool signal is noted in (a) filling the dilated right atrium, the small right ventricle, and right ventricular outflow tract. Thrombus is seen occupying the majority of the right ventricle. Blood pool signal is noted in (b) filling the right atrium and the right ventricle, which is now larger after thrombectomy. The normally functioning porcine bioprosthesis is noted at the level of the atrioventricular groove.

Figure 6 (a) Pre-operative and (b) post-operative magnetic resonance images – short axis views of the right and left ventricles. Blood pool signal is visualised in (a) filling the normal left ventricle (small arrow), but no blood pool signal is noted in the right ventricle. Blood pool signal is now noted in (b) filling both the right and left ventricles.
Ebstein’s malformation is a rare cardiac anomaly that is typically associated with severe tricuspid regurgitation, and dilatation of the right ventricle, functional and atrialised, and right atrium.Reference Morgan, Dearani and Danielson2 An interatrial communication is frequently present and atrial tachyarrhythmias are more common in the adult population. Echocardiography is the best initial diagnostic test and outlines tricuspid valve anatomy accurately. Magnetic resonance imaging is preferred to ascertain right ventricular dimensions and function. Operation consists of tricuspid valve repair or replacement,Reference Morgan, Dearani and Danielson2, Reference Dearani, Bacha and da Silva3 selective plication of the atrialised right ventricle, and right atrial reduction. An antiarrhythmia procedure, that is, “maze procedure”, is performed when indicated. In general, the interatrial communication is closed. The bidirectional cavopulmonary anastomosis, that is, superior vena cava to right pulmonary artery, is performed selectively and usually in the presence of severe right ventricular dilatation and/or dysfunction.Reference Quinonez, Dearani and Puga4 Early mortality is low in the current era (<5%). Late survival is good, but many patients do require reoperation in the future for recurrent tricuspid valve dysfunction.
Acknowledgments
We gratefully acknowledge Dr Joseph Kay from the University of Colorado for his excellent clinical management and guidance for the care given to the patient, as well as his assistance with providing high-quality echocardiographic figures. We also acknowledge Drs Niloufar Tibatabaei, and Naser Ammash for their excellent clinical management and guidance for the care given to the patient.








