The landmark event signalling the beginning of the history of the modern era of surgery for patients with congenitally malformed hearts was the successful ligation of a patent arterial duct in a seven year old child, performed by Robert Gross at the Boston Children’s Hospital in August, 1938.Reference Gross and Hubbard1 Less well known is the fact that John Streider, in Boston, had attempted surgical closure of a patent duct in a patient with fulminating bacterial endarteritis in 1937. The patient died on the fourth postoperative day, having suffered gastric distension and aspiration of vomit. Gross later advocated for division, rather than ligation of the duct. Also in 1938, Gross and Hufnagel commenced experiments anticipating surgical repair of coarctation of the aorta. They performed excision and end-to-end anastomosis in experimental animals, developing specialized arterial clamps and techniques for suturing. By the time they published the classic article describing their experimental work in 1945,Reference Gross and Hufnagel2 Clarence Craaford, in Stockholm, had accomplished the first repair of aortic coarctation, operating on the patient in October of 1944. The first repair of coarctation by Gross was performed in June 1945.
Early experience at Johns Hopkins
What is the relevance of these events, which took place shortly before or just after the initiation of surgical treatment of patients with tetralogy of Fallot? In a very general way, they describe the historical context of these pioneering accomplishments. More specifically, these events did not take place in isolation, but were very much intertwined, each one with the others. During this era, Helen Taussig was the physician in charge of the cardiology clinic at the Harriet Lane Home, the Pediatric Department of Johns Hopkins Hospital in Baltimore. Like others, she recognized that the principle crippling effect of tetralogy of Fallot was chronic and progressive hypoxaemia due to diminished flow of blood to the lungs. She also realized that her patients with this congenital cardiac malformation in association with persistent patency of the arterial duct experienced much less severe cyanosis than most other patients with tetralogy of Fallot. She hypothesized that creation of an arterial duct, or the equivalent of an arterial duct, would confer significant relief of the hypoxaemia of patients with tetralogy of Fallot, improving their functional capacity and longevity. She approached Robert Gross at Harvard with the proposal that, having accomplished surgical closure of persistently patent arterial ducts, he consider creation of an arterial duct. When Gross failed to show interest in the proposal, Taussig took her idea to Alfred Blalock who had recently returned from Vanderbilt University to head the Department of Surgery at Johns Hopkins. This collaboration between Blalock and Taussig was clearly an event that could aptly be described using the famous pronouncement of Louis Pasteur concerning scientific innovation: “Chance favors the prepared mind.” Blalock, and his paediatric colleague Edward Park, had proposed an operative procedure based upon turning down the divided distal end of the left subclavian artery functionally to bypass coarctation of the thoracic aorta. Perhaps more importantly, Blalock had been joined at Johns Hopkins by Vivien Thomas, the research assistant in his surgical laboratory at Vanderbilt. Despite not having had formal medical training, Thomas had become a skilled experimental surgical technician. Working in the laboratory of Blalock, first in Nashville and then in Baltimore, Thomas created canine models to study shock and other circulatory derangements. Importantly, in an effort to study pulmonary arterial hypertension, he developed a preparation in which the divided distal end of a branch of the aorta, either the subclavian or carotid artery, was connected to a pulmonary artery. While this model failed reliably to create pulmonary arterial hypertension, it did serve to increase the flow of blood to the lungs. Indeed, it was precisely the equivalent of the artificial duct sought by Taussig for her patients with cyanosis secondary to diminished pulmonary flow.

Figure 1 Portrait of Helen Taussig, by photographer Yousuf Karsh. Taussig was director of the pediatric cardiology clinic at Johns Hopkins. She noted the important beneficial effect of a persistent arterial duct in some of the patients with tetralogy of Fallot. (As published on the World Wide Web: www.hopkinshospital.org/…/Reading/history.html, reproduced with permission.)

Figure 2 Portrait of Alfred Blalock, by photographer Yousuf Karsh. In 1944, Blalock performed the first systemic to pulmonary arterial shunt to relieve profound cyanosis in a child with tetralogy of Fallot. He and his surgical team performed over 1000 such operations over the next few years. (As published on the World Wide Web: www.hopkinshospital.org/…/Reading/histroy.html, reproduced with permission.)
Thus, on November 29, 1944, Alfred Blalock performed the first operation on a cyanotic child aged one year with tetralogy of Fallot.Reference Blalock and Taussig3 Blalock was assisted by William Longmire. History records that Vivien Thomas, who had many times performed such operations in the animal laboratory, stood behind Blalock, watching over his shoulder and providing advice based upon his considerable surgical experience. The blue lips and fingertips of the baby became pink. This first operation, in a child with a left-sided aortic arch, was achieved by anastomosing the divided left subclavian artery to the left pulmonary artery. The next operation, in a patient with a right-sided aortic arch, consisted of anastomosis of the divided left-sided brachiocephalic artery to the left pulmonary artery. The third operation, in a patient with a left-sided arch, consisted of anastomosis of the divided right-sided brachiocephalic artery to the right pulmonary artery. Curiously, surgical educators, in their wisdom, describe the classic Blalock-Taussig shunt as consisting of anastomosis of the subclavian artery originating from the brachiocephalic artery to the pulmonary artery on the same side, which is the side opposite that of the aortic arch. So, in fact, none of the first three historic operations performed by Blalock on cyanotic patients was exactly the procedure that is considered today to be the classic operation. The palliative procedures had a high rate of success, and by 1950, 1000 such operations had been performed by Blalock and his team at Johns Hopkins, on children from cities and countries near and far. On the basis of their experience, Blalock and Taussig were invited to lecture at medical schools and hospitals around the world.

Figure 3 Photograph showing Alfred Blalock and his surgical team during one of the earliest operations on a cyanotic patient. This does not appear to be a photograph of the very first operation. Notably absent from the picture is Vivien Thomas, who watched over the shoulder of Blalock and gave advice during the initial procedure. Also, it appears that the first assistant surgeon in this picture is Denton Cooley. In the first operation, Blalock was assisted by William Longmire. (As published on the World Wide Web: www.hopkinshospital.org/…/Reading/history.html, reproduced with permission.)
Alternative approaches to palliation
In the same time frame, others, in places as close to Baltimore as Philadelphia, or as far away as London, pursued the objective of surgically increasing the flow of blood to the lungs in patients with tetralogy of Fallot, but based on a different principle, namely a direct surgical assault on the pulmonary valve and the muscle obstructing the right ventricular out flow tract, which together limit antegrade pulmonary flow. Two of the surgical pioneers who performed such procedures operated in two different hospitals in London, apparently without direct communication one with the other. The first such procedure was performed at the Middlesex Hospital, on December 4, 1947, on a patient of 20 years with tetralogy of Fallot. The surgeon was Thomas Holmes Sellors, later to become Sir Thomas. The report of this procedure appeared on June 26, 1948. Two weeks earlier, Russell Claude Brock, later to become the Lord Brock, reported three cases, performed at Guy’s Hospital.Reference Brock4 The report by Brock described the use of genitourinary sounds to probe and dilate the right ventricular outflow tract. He also designed a cardiotome specifically for such operations. His original concept had been to insert a cardioscope into the pulmonary trunk, and then pass it retrogradely through the pulmonary valve. Ultimately, however, he found it more practical to pass sounds or probes, and eventually a specifically designed valvulotome, in antegrade fashion through the muscle of the right ventricular outflow tract. One of the earliest patients of Brock, a child of four years of age, reportedly lived 43 more years without any additional surgery.Reference Gerlis, Smith and Somerville5 By June of 1950, Charles Bailey, of Hahnemann Hospital in Philadelphia, had performed such Brock Procedures on 8 patients with tetralogy of Fallot.Reference Downing, Bailey and Glover6
Other surgical pioneers of the time devised a variety of related closed procedures, either to address obstruction within the right ventricular outflow tract, or to increase the flow of blood to the lungs by creation of a systemic-to-pulmonary arterial shunt. In Chicago, Willis Potts created an anastomosis between the descending thoracic aorta and the left pulmonary artery.Reference Potts, Smith and Gibson7 In London, David Waterston proposed posterior anastomosis of the ascending aorta to the right pulmonary artery.Reference Waterston8 These side-to-side connections were touted as being more easily or predictably created in very small infants than was the shunt developed by Blalock and Thomas. Thousands of patients benefitted from these earliest palliative approaches. The operations were neither conceived, nor undertaken at the time, as a planned preliminary step in anticipation of an eventual intracardiac reparative operation.
The trend to intracardiac surgery
During the same period of time, surgeons were exploring the ultimate goal of intracardiac surgery. While research efforts to develop machines to bypass the heart or the heart and lungs were underway in several countries, surgeons were developing other clever ways to close intracardiac septal defects without such technology. Most of the techniques involved palpation of defects of the atrial septum by digital invagination of the right atrial appendage or free wall, followed by placement of sutures through the atrial wall and the margins of the defect. Tying down of the sutures was intended to obliterate the defect by approximating atrial appendage or atrial wall to the edges of the septal defect. This method, used successfully by Bailey and the Hahnemann group in Philadelphia, became known as the auricular-wall-cap. Another particularly imaginative and elegant technique involved temporary use of the so-called atrial well, and was developed by Robert Gross and Elton Watkins in Boston. Their technique, first developed in the experimental laboratory working with dogs, involved the use of a rubber funnel sutured to an incision in the right atrial wall, thus affording access to the interior of the right atrium and the septum. Since the well filled with blood, all intracardiac manoeuvres had to be guided by palpation rather than direct vision. Gross and his associates described their initial experience in the New England Journal of Medicine.Reference Gross, Pomeranz, Watkins and Goldsmith9 They reported experiments in 114 animals, and treatment of 6 patients, 2 with successful outcomes. This period of innovation was one in which several dozen surgical pioneers, in cities around the world, developed ingenious methods to create, and then repair, structural cardiac lesions, mostly septal defects in animals in the laboratory. Some went on to apply their techniques in patients. The few mentioned herein are no more than representative of some of the significant milestones in this quest.
The era of successful intracardiac surgery by direct vision was ushered in at the University of Minnesota on September 2, 1952. F. John Lewis closed an atrial septal defect in a five year old girl using total body hypothermia with inflow stasis. A cooling blanket was used to lower the temperature of the child to 82 degrees Fahrenheit. After the defect was closed, the patient was rewarmed in a tank of warm water. This first successful case was followed by more than 50 operations performed by Lewis to close defects in the atrial septum during the next three years. In an important history of the early days of heart surgery at the University of Minnesota, Vincent L. Gott provides the following anecdote,Reference Gott10 recounting that, on September 2, 1952, there stood behind F. John Lewis in the operating room a young surgeon on the faculty of the University of Minnesota named Walt Lillehei. He was heard to say as he left the room, “Boy, there’s got to be a better way to do open-heart surgery than with total body hypothermia.”
The era of open heart surgery
Eight months after the landmark achievement of Lewis, John Gibbon successfully closed an atrial septal defect in an 18 year-old girl at Jefferson Medical College in Philadelphia. He used the heart lung bypass machine and screen oxygenator that he and his research team had developed during many years in the laboratory there, and in Boston. The monumental procedure was performed on May 15, 1953.Reference Gibbon11 Gibbon went on to attempt repairs in 4 additional patients who were diagnosed as having atrial septal defects. None survived, and the discouraged pioneer never again performed open heart surgery. According to Gott,Reference Gott10 a pervasive pessimism was prevalent, with surgeons adapting the view that these patients suffered from a so-called sick heart syndrome, and that they would need to be supported on a heart-lung machine for days after surgery.
This general pessimism was not shared by C. Walton Lillehei, the surgeon who had witnessed the first intracardiac repair performed in Minnesota under total body hypothermia. He set out to find a better method. With his colleagues, he had performed experiments in dogs in which a support animal was used to pump oxygenated blood into the circulation of the animal representing the patient, maintaining adequate perfusion and delivery of oxygen. Flow could be assisted by a mechanical pump, but no artificial oxygenator was required. On March 26, 1954, Lillehei, Varco, Warden, and Cohen used such cross-circulation to carry out the first ever closure of a ventricular septal defect.Reference Warden, Cohen, Read and Lillehei12 The patient was a desperately ill boy of one year. The father of the child served as the biologic oxygenator. As an intern, Gott observed the surgery, as did a third year medical student named Norman Shumway. Sadly, the child died of pneumonia 11 days after surgery. But the next two children put forward for intervention had successful repairs of ventricular septal defects using controlled cross-circulation with support provided by a parent. On August 31, 1954, Lillehei performed the first successful repair of tetralogy of Fallot using this technique. Unique among the early cases, this operation involved not a parent or relative of the young patient as the supporter, but a circulatory donor with compatible ABO blood group who was reportedly recruited by the Red Cross. The patient, aged 11 years, made an excellent recovery. During the next year, Lillehei and his associates performed open heart surgery on 45 children using cross circulation. Of these patients, 10 had tetralogy of Fallot, including one with pulmonary atresia.Reference Lillehei, Cohen and Warden13 The last 3 of these patients underwent closure of the ventricular septal defect with a patch, as opposed to direct closure with sutures, as had been done in the earlier patients. The operations were performed on a beating heart, without a cardiotomy suction. The support times were all under 21.5 minutes, including successful repair of tetralogy with pulmonary atresia in a child of 22 months. Of the 10 patients undergoing repair of tetralogy of Fallot in this fashion during a period of 13 months, 6 were discharged alive from the hospital, including the patient with pulmonary atresia. The general pessimism concerning open heart surgery had been challenged, and vanquished.
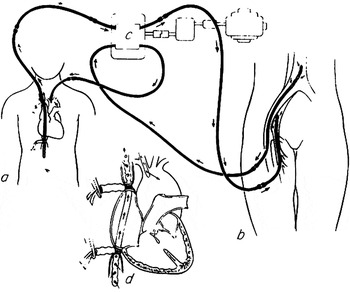
Figure 4 Schematic drawing of the system of “controlled cross circulation” devised and used by C. Walton Lillehei and his surgical team at the University of Minnesota to perform the first intracardiac repairs of ventricular septal defects and tetralogy of Fallot. The parent of the patient, or another “circulation donor” served as the biological oxygenator. Reproduced with permission of the Society of Thoracic Surgeons [Ann Thorac Surg 1986; 41: 12].
In July 1954, Clarence Crafoord, in Stockholm, successfully resected a left atrial myxoma, using a heart lung machine with disc oxygenator that had been developed by Ake Senning. On March 22, 1955, John Kirklin, working at the Mayo Clinic in Rochester, Minnesota, performed successful intracardiac repair of a ventricular septal defect using a mechanical pump oxygenator named the Mayo-Gibbon oxygenator. In 1956, the team headed by Kirklin reported on a series of 20 patients with large ventricular septal defects who had undergone intracardiac repair using the mechanical pump oxygenator.Reference DuShane, Kirklin and Patrick14 The first successful repair of a patient with tetralogy of Fallot using this approach was achieved by Kirklin at the Mayo Clinic in 1955. He used normothermic rates of flow of 70 millilitres per kilogram per minute, along with a pump sucker to return intracardiac blood to the machine. Surgical methodology evolved quickly. Warden, Lillehei, and colleagues introduced the use of a patch to enlarge the right ventricular infundibulum,Reference Warden, DeWall, Cohen, Varco and Lillehei15 and in 1959, Kirklin and his colleagues reported the use of patching across the ventriculo-pulmonary junction.Reference Kirklin, Ellis, McGoon, DuShane and Swan16 The group from the Mayo Clinic then reported, in 1965, the use of a conduit placed from the right ventricle to the pulmonary arteries for repair of tetralogy of Fallot with pulmonary atresia,Reference Rastelli, Ongley, Davis and Kirklin17 with Donald Ross and colleagues in London being the first to report the use of a valved extracardiac conduit for this purpose in 1966.Reference Ross and Somerville18

Figure 5 Photograph of the Mayo-Gibbon pump oxygenator. This apparatus was used by John W. Kirklin at the Mayo Clinic to perform the first repairs of tetralogy of Fallot that were accomplished using mechanical cardiopulmonary bypass. (As published on the World Wide Web: www.mayoclinicproceedings.com/images/8005/800, reproduced with permission.)
Conclusions
So many of the surgical concepts integral to present day surgical management of patients with tetralogy of Fallot, therefore, were conceptualized and validated by visionary surgical pioneers four and five decades ago. Of similar significance is the fact that so many of the basic strategies for cardiac surgery in general were developed by these pioneers while performing surgical procedures in, and caring for, patients with tetralogy of Fallot.







