Introduction
Hypertrophic cardiomyopathy is characterised by asymmetric left ventricular hypertrophy in conjunction with variable degrees of left ventricular outflow tract obstruction. While severe obstruction is associated with syncope, arrhythmias, and sudden death in adults, such events are probably less common in children with hypertrophic cardiomyopathy,Reference Maron, Rowin and Casey 1 and appear to occur less frequently compared with children with other subtypes of cardiomyopathy.Reference Bharucha, Lee and Daubeney 2 Nonetheless, progressive left ventricular outflow tract obstruction is frequently a cause for concern in the paediatric population with hypertrophic cardiomyopathy. In adults, procedures such as septal myomectomy and alcohol septal ablation may be considered in the face of worsening left ventricular outflow tract obstruction; however, experience with these interventions in children is limited and indications for intervention in children are not well established. Therefore, pharmacologic methods of reducing left ventricular outflow tract obstruction remain the mainstay of hypertrophic cardiomyopathy management in children.
Three classes of medications are widely used in hypertrophic cardiomyopathy for the purposes of left ventricular outflow tract gradient reduction: beta-blockers, calcium channel-blockers, and disopyramide. Beta-blockers (e.g. propranolol) primarily reduce the left ventricular outflow tract gradient through reduction of heart rate and commensurate increase in the relative duration of diastole. Calcium channel blockers, e.g. verapamil, act as a negative inotrope, thereby decreasing cardiac output and by extension the left ventricular outflow tract gradient; however, their use in children is limited, possibly related to the perceived risk of profound depression of myocardial contractility with intravenous administration in very young children.Reference LaPage, Bradley and Dick 3 Disopyramide, a class IA antiarrhythmic, is a cardiac sodium channel blocker that also has negative inotropic properties.Reference Sherrid, Pearle and Gunsburg 4 Studies in adults with hypertrophic cardiomyopathy have reported favourable reductions in left ventricular outflow tract gradients in patients demonstrating persistently elevated left ventricular outflow tract gradients despite therapy with beta-blockers and calcium channel blockers.Reference Sherrid and Arabadjian 5 – Reference Sherrid, Shetty and Winson 7 Use of disopyramide in hypertrophic cardiomyopathy appeared to be safe in these studies. There is little reported experience, however, regarding the use of disopyramide for the reduction of left ventricular outflow tract gradients in children with hypertrophic cardiomyopathy, with only two published case series to our knowledge.Reference Teraguchi, Ikemoto and Kobayashi 8 , Reference Duncan, Tyrrell and Bharadwaj 9 Given this relative paucity of data, the purpose of this study is to report our centre’s experience with disopyramide in children with hypertrophic cardiomyopathy in a recent cohort, with a focus on efficacy and adverse effects.
Materials and methods
This study was reviewed and approved by the institutional review board of The Children’s Hospital of Philadelphia with waiver of informed consent. The medical records of all patients ⩽21 years of age treated with disopyramide for left ventricular outflow tract obstruction in the setting of hypertrophic cardiomyopathy at The Children’s Hospital of Philadelphia between 1/1/2007 and 9/30/2015 were reviewed retrospectively. Patients were excluded only if they were adults (>21 years) at the time of disopyramide initiation. Demographic and clinical data were abstracted from the medical record, including age at disopyramide initiation, gender, primary diagnosis, other associated diagnoses, weight at disopyramide initiation, dose administered, transcatheter or surgical interventions for hypertrophic cardiomyopathy, electrocardiogram data, and echocardiographic data. The primary outcome measure was comparison of the left ventricular outflow tract Doppler-derived peak instantaneous gradient, obtained by transthoracic echocardiography, before initiation of disopyramide to the lowest Doppler-derived gradient while on disopyramide. All patients were admitted to the hospital for initiation and dose titration of disopyramide, with daily electrocardiogram monitoring.
Echocardiograms were obtained without sedation, with digital cine and still images archived on Syngo Dynamics version 9.5 (Siemens, Erlangen, Germany). Left ventricular outflow tract gradients were reviewed and recorded by a single trained echocardiographer (M.J.O.). The highest peak instantaneous gradient was obtained via direct interrogation of the left ventricular outflow tract, using continuous wave Doppler from subcostal, apical, or parasternal views. Interrogation of the mitral regurgitation jet velocity enabled estimation of left ventricular pressure, which was used to confirm the validity of left ventricular outflow tract Doppler results. Because patients may have had multiple echocardiograms performed following initiation of disopyramide, both the most recently available gradient and the lowest attained gradient were analysed. In addition to assessment of Doppler-derived echocardiographic left ventricular outflow tract gradient changes, secondary endpoints included description of adverse events and patient outcomes – alive, death, and heart transplant.
Data were analysed using standard summary statistics, with comparison of Doppler-derived left ventricular outflow tract gradients achieved by paired Student’s t-test. Data are presented as median and range unless otherwise noted. Stata 9.0 was used for all data analysis.
Results
Nine patients with hypertrophic cardiomyopathy received disopyramide during the study period. The median age at disopyramide initiation was 5.6 years (6 days–12.9 years). Two of the nine patients (22.2%) were infants, aged 6 days and 133 days, respectively. Seven of the nine patients (77.8%) were male. Five of the nine patients (55.6%) had idiopathic hypertrophic cardiomyopathy, whereas four had phenotypic features consistent with Noonan syndrome. Genetic testing for sarcomeric mutations known to be associated with hypertrophic cardiomyopathy, or mutations associated with Noonan/LEOPARD phenotype, was performed in eight of nine patients, with two patients having pathogenic mutations known to be associated with hypertrophic cardiomyopathy (MYL2 and PTPN11), four patients having variants of unknown significance, and two patients with negative results. The indication for disopyramide in all patients was persistent, progressive left ventricular outflow tract obstruction despite therapy with beta-blockers and/or calcium channel blockers. At the time of disopyramide initiation, all patients were receiving a beta-blocker; two patients had undergone prior surgical septal myomectomy. Median follow-up for all patients was 2.5 years (range 15 days–10.4 years); median duration of therapy with disopyramide was 1.6 years (range 12 days–10.4 years). A summary of demographic and clinical data of the patients is presented in Table 1.
Table 1 Demographic and clinical characteristics of the patient population.

F=female; HCM=hypertrophic cardiomyopathy; LVOT=left ventricular outflow tract; M=male; OHT=orthotopic heart transplantation; RV=right ventricle; VUS=variant of unknown significance
The target dose of disopyramide was 10 mg/kg/day divided three or four times daily. The median initial dose of disopyramide was 10 mg/kg/day (range 1.5–13.4 mg/kg/day), divided three or four times daily, with the four times daily dosing typically reserved for infants and young children. The median final dose of disopyramide was 9 mg/kg/day (range 5.6–12.7 mg/kg/day).
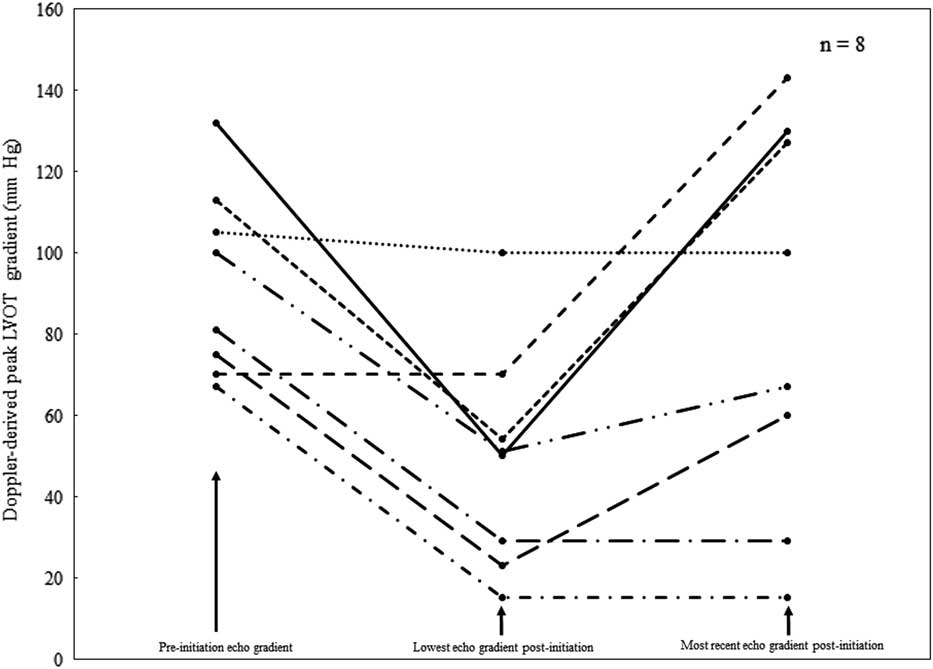
Before initiation of disopyramide, the median left ventricular outflow tract peak instantaneous gradient in the patient population was 81 mmHg (range 30–132 mmHg). Eight of the nine patients continued disopyramide for sufficient time to have post-initiation echocardiograms performed. In these eight patients, the median % maximal reduction in the left ventricular outflow tract Doppler gradient when compared with the median left ventricular outflow tract Doppler gradient before initiation of disopyramide was 58.2%; however, the median left ventricular outflow tract Doppler gradient at most recent follow-up while still on disopyramide was 84 mmHg (range 15–143 mmHg), representing an actual increase in the left ventricular outflow tract Doppler gradient. The median time elapsed between initiation of disopyramide and the most recent follow-up echocardiogram was 715 days (range 12–3686 days). Using paired Student’s t-test, the maximum % reduction in left ventricular outflow tract Doppler gradient after initiation of disopyramide was significant (p=0.002), but not when comparing pre-initiation gradient with the gradient at most recent follow-up while still on disopyramide. Altogether, seven of eight patients experienced a reduction in left ventricular outflow tract Doppler gradient after initiation of disopyramide (see Fig 1).

Figure 1 Graphical representation of changes in Doppler-derived left ventricular outflow tract gradients over time in eight patients taking disopyramide for hypertrophic cardiomyopathy. Three points in time are demonstrated: the Doppler-derived left ventricular outflow tract gradient before disopyramide initiation, the lowest obtained left ventricular outflow tract gradient after disopyramide initiation, and the most recently obtained left ventricular outflow tract gradient while still on disopyramide. echo=echocardiogram; LVOT=left ventricular outflow tract.
During the follow-up period, disopyramide was discontinued in six of the nine patients. Four patients underwent septal myomectomy for increasing left ventricular outflow tract gradients, one patient underwent heart transplantation, and one patient discontinued disopyramide owing to side effects (emesis). Side effects were noted in three of nine patients and included emesis (two patients) and gingival hyperplasia (one patient). Electrocardiogram monitoring during initiation and maintenance of disopyramide therapy revealed a statistically significant (p=0.004) but likely clinically insignificant increase in the corrected QT interval: median 440 msec (range 393–505 ms) at initiation to median maximum 488 ms (range 473–616 ms), with a median % increase of 12.9%. However, no known arrhythmias occurred. There were no episodes of new QRS widening and/or bundle branch block.
At the time of most recent follow-up, eight of nine patients were alive, with a median follow-up duration of 2.5 years (range 636 days–9.8 years). One patient, a neonate with severe hypertrophic cardiomyopathy and left ventricular outflow tract obstruction, underwent heart transplantation but died in the early post-transplant period from primary graft failure.
Discussion
In this small series of children with hypertrophic cardiomyopathy, we found that disopyramide was generally effective in initially reducing Doppler-derived left ventricular outflow tract gradients, with a decrease in the left ventricular outflow tract gradient in seven of the eight patients in whom pre- and post-initiation echocardiograms were performed. In addition, disopyramide appeared safe in this small series, with no serious side effects or new arrhythmias. We therefore propose that disopyramide be considered in children with severe hypertrophic cardiomyopathy and increasing left ventricular outflow tract gradients despite the highest tolerated doses of beta-blockade, before considering more invasive therapies such as septal myomectomy. To the best of our knowledge, this is the largest series in the literature to date examining the use of disopyramide in children with hypertrophic cardiomyopathy.
In adults with hypertrophic cardiomyopathy, disopyramide has been shown to be effective at reducing left ventricular outflow tract gradients in symptomatic patients with hypertrophic cardiomyopathy. In a study of 221 patients who received disopyramide, a 60% reduction in left ventricular outflow tract gradient was noted. Furthermore, of those patients who received disopyramide, nearly two-thirds were able to avoid invasive procedures to reduce the left ventricular outflow tract gradient.Reference Sherrid, Shetty and Winson 7 Our finding of a median 58% maximal reduction in the left ventricular outflow tract gradient in our patient population is very similar to that obtained by the investigators in the aforementioned study. Interestingly, disopyramide use in children and young adults with hypertrophic cardiomyopathy appears to be uncommon. In a recent study of 474 patients with hypertrophic cardiomyopathy aged 7–29 years cared for at two referral centres, only 16 (3.4%) were receiving disopyramide, despite approximately 25% of the population having a septal thickness ⩾30 mm and 30% having a family history of hypertrophic cardiomyopathy-related death.Reference Maron, Rowin and Casey 1
The list of potential adverse effects from disopyramide is lengthy, and ranges from minor issues such as gastrointestinal upset to more serious adverse events, such as prolongation of the QT interval and an increase in predisposition to arrhythmias. While an aggregate increase of ~40 ms in the QTc interval was noted in our study, we did not encounter any documented arrhythmias associated with the use of disopyramide. Nonetheless, four out of the nine patients in our study had QTc intervals above 500 ms, a common threshold for concern regarding arrhythmia potential. For this reason, we recommend that all patients be admitted to a telemetry unit for initiation and/or dose titration of disopyramide, with daily electrocardiogram monitoring. Although this study was not intended to establish pharmacokinetic or pharmacodynamic norms for disopyramide in children with hypertrophic cardiomyopathy, in those patients who responded to therapy dosing was within previously published studies in children – approximately 5–15 mg/kg/day divided three or four times daily.Reference Baker, Hayler and Curry 10
Although we report effective left ventricular outflow tract gradient reduction in this series of patients, optimism regarding the durability of reduction should be tempered by the fact that the magnitude of gradient reduction appeared to decrease over time, as evidenced by our finding that the median maximum % gradient reduction of 57.2% (maximum % reduction) had actually increased slightly at most recent follow-up while still taking disopyramide. This trend is also shown in the Figure 1. Furthermore, disopyramide was discontinued in four patients who ultimately underwent myomectomy, indirectly indicating that disopyramide was not sufficient for left ventricular outflow tract gradient control in these patients. In addition, one patient underwent myomectomy but continued disopyramide therapy post myomectomy. These observations likely reflect progression of disease in a selected population with clinically severe disease or, less likely, inadequate disopyramide dosing or tachyphylaxis to the drug itself. Of additional note, one patient with Noonan’s syndrome phenotype with biventricular hypertrophy and biventricular outflow tract obstruction underwent right ventricular myomectomy for ongoing disease despite disopyramide, which suggests that disopyramide may not be effective for right-sided outflow tract obstruction. This is not altogether surprising, given that the pathophysiologies of left- and right-sided outflow tract obstruction are likely quite different. These concerns regarding the beneficial effect of disopyramide in the long term notwithstanding, the use of disopyramide in our patient population likely deferred the time at which surgery was deemed to be clinically necessary; however, this cannot be assessed definitively with a retrospective study design.
The potential utility of disopyramide in deferring the clinical need for invasive therapies in children with hypertrophic cardiomyopathy warrants a brief discussion of the indications and outcomes of these therapies in the paediatric hypertrophic cardiomyopathy population. Currently, septal myomectomy is uncommonly performed in children, with many centres having little, if any, experience with its application in children. At present, alcohol septal ablation is not recommended in the paediatric population with hypertrophic cardiomyopathy owing to concerns regarding the long-term implications of septal scar formation in patients whose life expectancy can be measured in decades, rather than years.Reference Gersh, Maron and Bonow 11 The indications for septal myomectomy in children with hypertrophic cardiomyopathy remain unclear. Select centres have reported favourable outcomes for septal myomectomy in patients as young as neonates, with successful intervention possibly altering the natural history of the disease.Reference Altarabsheh, Dearani and Burkhart 12 – Reference Poterucha, Johnson and O’Leary 14 While attempts to medically temporise the progression of disease using beta-blockers and disopyramide seem to be reasonable and effective in the short-term, surgical intervention should not be delayed in children with progressive gradients and/or symptoms attributable to obstruction, left ventricular diastolic dysfunction, mitral regurgitation, or arrhythmias. However, it should be recognised that symptoms of left ventricular outflow tract obstruction may be challenging to elicit in infants and young children, thus complicating decision-making when considering patients for surgery. Given the inherent ambiguity in assessing symptomatology related to left ventricular outflow tract obstruction, consideration of temporising medical therapy with disopyramide seems appropriate.
This study has several important limitations, foremost of which is the small sample size. It should be stressed that the patients reported here represent a higher-risk, selected population owing to the severity of outflow tract obstruction. However, our patient population was skewed towards a younger age group, a group in which invasive strategies to reduce left ventricular outflow tract gradients are less commonly used, and for whom additional medical therapies are needed. Hypertrophic cardiomyopathy in children, particularly young children, is a heterogeneous disease with a sizeable proportion of patients having hypertrophic cardiomyopathy associated with genetic syndromes or inborn errors of metabolism, which may have different clinical courses and responses to therapy than idiopathic hypertrophic cardiomyopathy. Finally, the use of an imaging surrogate, Doppler-derived left ventricular outflow tract gradient, for disopyramide efficacy has obvious limitations, given that the Doppler-derived gradient may be dynamic and highly dependent on heart rate, cardiac output, and ventricular loading conditions.
In this small series of children, disopyramide appeared to be effective in the initial reduction of Doppler-derived left ventricular outflow tract gradients in patients with hypertrophic cardiomyopathy; however, evidence of long-term benefit is lacking. Nonetheless, these data suggest that disopyramide can be safely used in children of all ages with hypertrophic cardiomyopathy to reduce left ventricular outflow tract gradients before consideration of more invasive therapies such as myomectomy. Further studies of disopyramide in children with hypertrophic cardiomyopathy are needed to establish populations in whom the greatest benefit will be obtained.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the United States on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committee of The Children’s Hospital of Philadelphia.