Background
Kawasaki disease mainly affects infants and young children and is characterised by systemic vasculitides accompanied by acute fever.Reference Burns and Glodé 1 , Reference Kawasaki, Kosaki, Okawa, Shigematsu and Yanagawa 2 Approximately 20–25% of patients without treatment develop a coronary arterial lesion, the primary complication of Kawasaki disease.Reference Lin, Sun, Wu, Wang, Lue and Wu 3 This disease is the leading cause of acquired heart disease in children in developed countries,Reference Makino, Nakamura and Yashiro 4 , Reference Heuclin, Dubos and Hue 5 and thus decreasing the risk for formation of coronary arterial lesions in patients with Kawasaki disease is critical. Previous studies have reported that intravenous immunoglobulin therapy within 10 days of symptom onset is the most timely therapy for decreasing the risk for coronary arterial lesions to 5–10%.Reference Ho, Fu, Lin and Jan 6 , Reference Patel and Shulman 7 Intravenous immunoglobulin has been reported to inhibit the inflammatory reaction, reduce the expression of cytokines and growth factors, activate the complement system, and control T-cell and macrophage activation.Reference Ballow 8 It has also been suggested that intravenous immunoglobulin decreases the chemotactic migration of monocytes and the occurrence of coronary arterial lesions by inhibiting nuclear factor-κ B and P38 mitogen-activated protein kinase, and by reducing the expression and activity of matrix metalloproteinase-9.Reference Zhou, Huang, Xie, Shen, Xiao and Wang 9 Although many studies have reported the anti-inflammatory function of intravenous immunoglobulin therapy, the underlying mechanisms are not completely understood.
Several cell types, including inflammatory cells, are known to deliver exosomes to the extracellular space.Reference Pap, Pállinger, Pásztói and Falus 10 Exosomes are 30–100-nm membrane vesicles that arise from the fusion of early endosomes and multivesicular bodies in cells.Reference Lakkaraju and Rodriguez-Boulan 11 , Reference Mathivanan, Ji and Simpson 12 Exosomes are important intercellular communicators and appear in all body fluids.Reference Yoon, Kim and Gho 13 , Reference Inal, Kosgodage, Azam, Stratton, Antwi-Baffour and Lange 14 They contain proteins involved in fission, scission, and vesicular transport of selective messenger RNAs and microRNAs with functional activation.Reference Mathivanan, Ji and Simpson 12 , Reference Kosaka, Iguchi, Yoshioka, Takeshita, Matsuki and Ochiya 15 The binding or uptake of exosomes by target cells can markedly alter the activities of these target cells; for example, exosomal proteins modulate T-cell activation and immunosuppression by initiating signal transduction and gene transcription in these cells.Reference Hwang and Ki 16 Therefore, investigating the changes in the proteomic profile of serum exosomes could provide insights into the pathological processes of diseases as well as enable the identification of potential markers for the diagnosis and treatment of such diseases.
In this study, we used proteomics analysis to evaluate the global expression profile of serum exosomal proteins before and after intravenous immunoglobulin therapy in patients with Kawasaki disease. The changes in the identified differentially expressed proteins and the corresponding pathway assessments will be helpful for disclosing the effects of intravenous immunoglobulin therapy on the proteomic profile of serum exosomes in patients with Kawasaki disease.
Methods
Sample collection and preparation
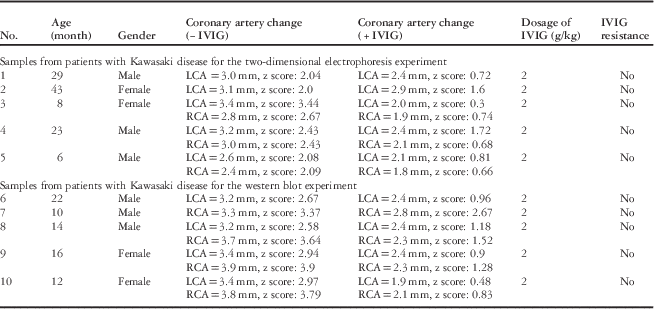
This study was approved by the Ethics Committees at Guangzhou Women and Children’s Medical Center [2013]077, and written informed consent was obtained from the guardians of all donors. The children hospitalised in Guangzhou Women and Children’s Medical Center in 2015 with a definite diagnosis of Kawasaki disease with coronary artery dilation as evidenced by z scores were recruited into this study (Table 1). The diagnosis was according to the American Heart Association and the Japanese Ministry of Health and Welfare criteria. Serum samples from 10 patients with Kawasaki disease were collected before and after intravenous immunoglobulin therapy. These patients were diagnosed on the 5th day and intravenous immunoglobulin therapy was given on the same day. After intravenous immunoglobulin therapy for 30 days, a second round of echocardiographies were performed, and patients recovered from Kawasaki disease symptoms. The clinical data of the patients with Kawasaki disease before and after intravenous immunoglobulin therapy are listed in Table 1. In addition, blood samples from 10 sex- and age-matched healthy children were collected as controls. A total of five samples from each group were pooled together for two-dimensional electrophoresis experiments, and another five serum samples from each group were used individually for western blot experiments for verification. All blood samples were centrifuged at 1000 g for 10 minutes, and the supernatants were stored at −80°C.
Table 1 Clinical data of 10 patients with Kawasaki disease before and after intravenous immunoglobulin therapy.
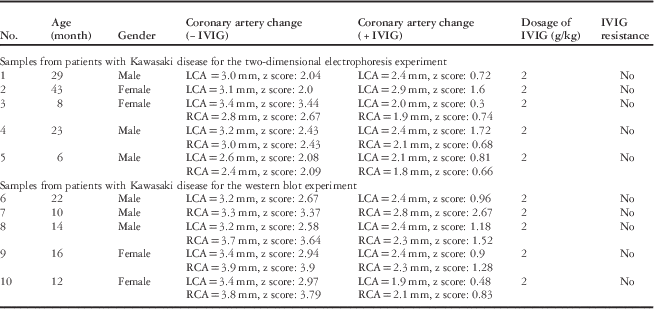
−IVIG=before intravenous immunoglobulin therapy; +IVIG=after intravenous immunoglobulin therapy; LCA=left coronary artery; RCA=right coronary artery
Isolation of serum exosomes
The preserved supernatants of blood samples were used for exosome extraction by means of ExoQuick precipitation (System Biosciences Inc., Mountain View, California, United States of America) according to the manufacturer’s instructions. The exosomes from five patients – equal amounts of each sample – and from five healthy children were pooled together for protein extraction. Protein concentration was then determined using the Bradford protein assay kit (Bio-Rad, Hercules, California, United States of America).
Characterising of exosomes by transmission electron microscopy
The extracted exosomes from the serum were centrifuged at 1500 g at 4°C for 10 minutes. The pellets were suspended in phosphate buffered saline at four times the volume of serum. Thereafter, the exosome suspension (100 μl) was poured onto a copper mesh and placed on a wax plate for 4 minutes. The copper mesh was subsequently placed in 2% phosphotungstic acid for 5 minutes and then on a filter paper for drying. Finally, the exosomes were visualised using transmission electron microscopy to characterise their morphological features.
Western blot analysis
After lysis, the protein samples of the exosomes were separated using 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis, electrophoresed, and transferred onto a polyvinylidene fluoride membrane. Blots were detected with several antibodies, including CD9, flotillin, complement C3, apolipoprotein A-IV, insulin-like growth factor-binding protein complex acid labile subunit, and β-actin antibodies (Proteintech Group, Rosemont, Illinois, United States of America), following the standard protocol. All immunoblot detections were performed with horseradish peroxidase-conjugated secondary antibodies (Proteintech Group) and chemiluminescence (Pierce, Rockford, Illinois, United States of America). The quantitative analysis of blots was performed using Image J software.
Two-dimensional electrophoresis analysis
The protocol for two-dimensional electrophoresis assay was described in detail in a previous study.Reference Zhou, Yuan and Zhao 17 In brief, the above prepared protein samples (200 μg) from five patients with Kawasaki disease before and after intravenous immunoglobulin therapy and those from healthy children were used for the two-dimensional electrophoresis assay. First, immobilized pH-gradient strips (pH 4–7, 24 cm; GE Healthcare, Piscataway, New Jersey, United States of America) were selected to separate proteins at the following settings: at 300 V for 20 minutes, at 700 V for 30 minutes, at 1500 V for 1.5 hours, at 9000 V for 3 hours, and at 9000 V for 4 hours. After isoelectric focussing, the strips were incubated with an immobilised pH-gradient equilibration buffer containing 6 M urea, 2% sodium dodecyl sulphate, 30% glycerol, 0.375 M Tris, 20 mg/ml dl-Dithiothreitol, and a trace of bromophenol blue for 15 minutes, and was alkylated for another 15 minutes. The equilibrated gel strip was placed on top of a 12.5% sodium dodecyl sulphate–polyacrylamide gel electrophoresis gel and sealed with 0.5% agarose containing a little bromophenol blue. Sodium dodecyl sulphate–polyacrylamide gel electrophoresis was carried out at a constant current of 15 mA for 30 minutes per gel and then at 30 mA per gel until the bromophenol blue reached the bottom of the gels. Proteins were detected by means of silver nitrate staining. Silver staining was used to observe the protein spots, and the gels were imaged with a scanner. ImageMaster 2D Platinum 5.0 software (GE Healthcare, Piscataway, New Jersey, United States of America) was employed to analyse the gels, which calculated the volumes of spots relative to the background. The expression levels of proteins were normalised according to the ratio of specific spot volume to total volume of spots on the gels. For further analysis, we chose the protein spots with expressional levels larger than 1.5-fold compared with the control group.
Tryptic in-gel digestion and matrix-assisted laser desorption ionisation time of flight/time-of-flight mass spectrometry analysis
Tryptic in-gel digestion of the selected protein spots was carried out according to the method described in a previous study.Reference Zhou, Yuan and Zhao 17 After digestion, the materials were analysed with ABI 4800 matrix-assisted laser desorption ionisation time of flight/time-of-flight mass spectrometry (Applied Biosystems, Foster, California, United States of America). Raw data from the mass spectrometer were automatically processed with Mascot v2.1 software and the UniProt database with the taxonomy of Homo sapiens with the settings including trypsin enzyme, fixed modification with carbamidomethylation, and variable modification with oxidation (M). The Mascot search routine revealed that a protein score greater than 71 was required per protein.
Protein categorisation and protein–protein interaction network constructions
The interaction network of differentially expressed proteins was automatically constructed with ReactomeFIViz, a Cytoscape app to assist biologists in pathway enrichment and network-based data analysis.Reference Wu, Dawson, Duong, Haw and Stein 18 Further, ReactomeFIViz can provide gene signatures from gene expression data sets. The biological processes and molecular functions of differentially expressed proteins were enriched through ReactomeFIViz and the Cytoscape platform based on Gene Ontology terms. The corresponding p-value analysis was simultaneously obtained using ReactomeFIViz.
Results
Isolation and confirmation of serum exosomes
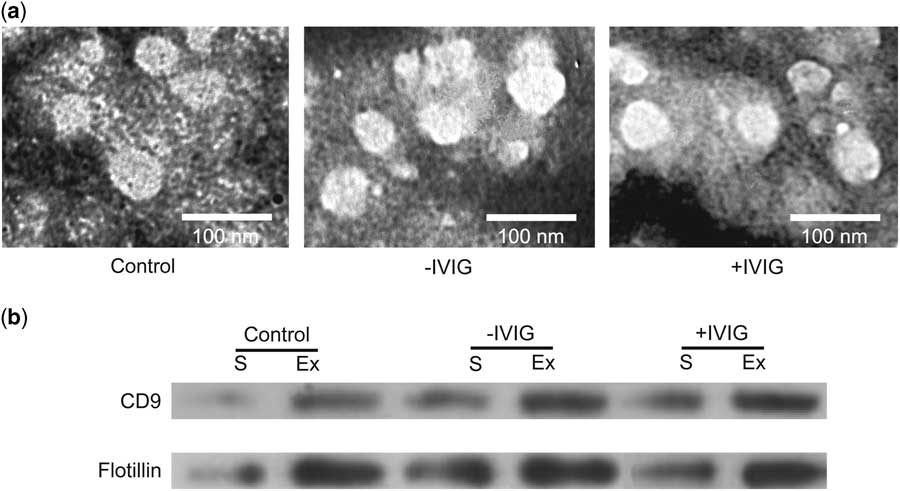
To identify the differential proteome of serum exosomes from patients with Kawasaki disease after intravenous immunoglobulin therapy, we first isolated exosomes before and after intravenous immunoglobulin therapy from the serum of patients with Kawasaki disease and from healthy controls. Transmission electron microscopy images showed that the microvesicles were spherical structures, ~30–100 nm in diameter (Fig 1a), consistent with the previously reported size of exosomes. Following this, western blot was used to detect the expression of exosomal markers, including CD9 and flotillin proteins. The expression of these two proteins was remarkably higher in the microvesicles than in the serum (Fig 1b), thus confirming the existence of exosomes.
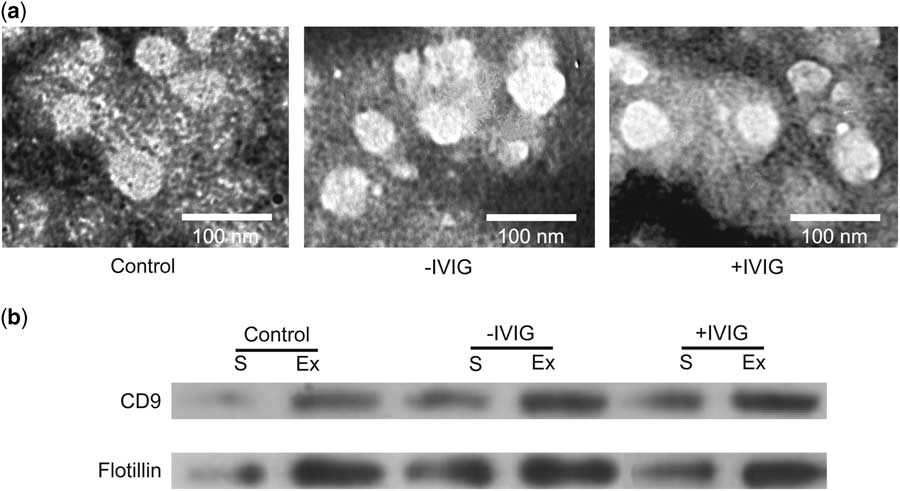
Figure 1 Isolation of serum (S) exosomes (Ex). ( a ) Transmission electron microscopic images of Ex isolated from healthy children (Control) and from patients with Kawasaki disease before intravenous immunoglobulin therapy (−IVIG) and after intravenous immunoglobulin therapy (+IVIG). ExoQuick precipitation was used to isolate Ex. Scale bar: 100 nm. ( b ) Western blot results for CD9 and flotillin expression in sera and Ex fractions.
The differentially expressed proteome of serum exosomes after intravenous immunoglobulin therapy
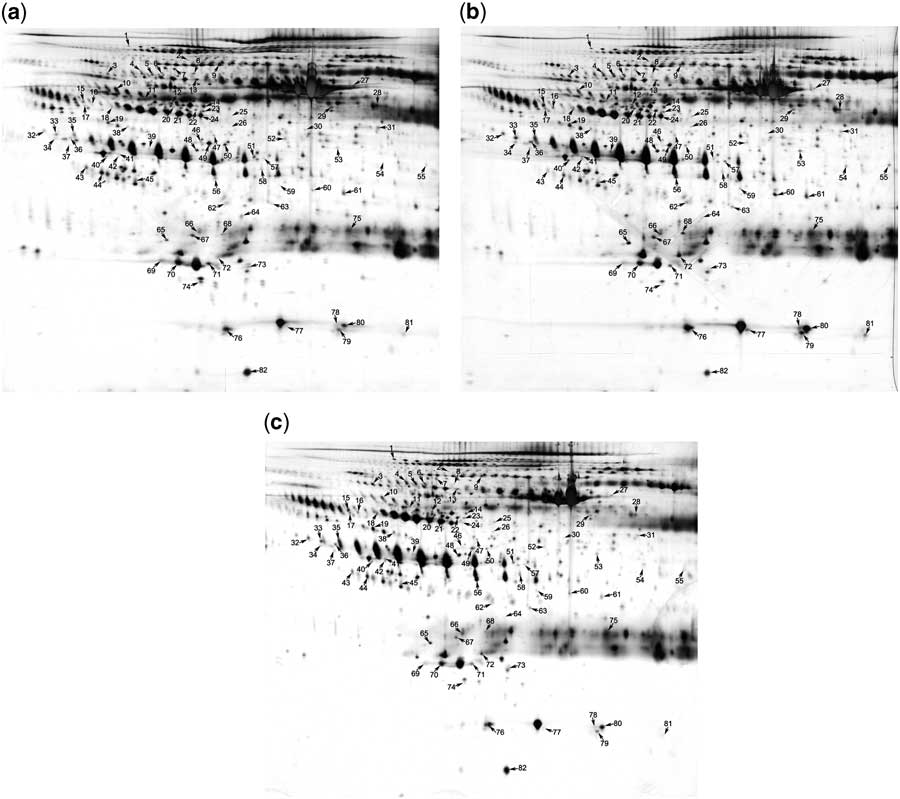
Next, we used two-dimensional electrophoresis combined with matrix-assisted laser desorption ionisation time of flight/time-of-flight mass spectrometry analysis to identify the differentially expressed serum exosomal proteins before and after intravenous immunoglobulin therapy. For each group, exosomes were isolated from pooled serum samples of five children and lysed. The exosomal proteins were subjected to standard two-dimensional electrophoresis. As shown in Figure 2, 82 protein spots, identified using two-dimensional electrophoresis, were selected for mass spectrometric analysis. The differential proteins between serum exosomes from healthy children and patients with Kawasaki disease before intravenous immunoglobulin are listed in Supplementary Table S1. It includes 69 protein spots, of which 22 were downregulated and 47 were upregulated, with changes larger than 1.5-fold. Supplementary Table S2 presents the 59 differentially expressed protein spots between the serum exosomes of patients after intravenous immunoglobulin therapy and those of healthy children. Of these, 29 were downregulated and 30 were upregulated. Because of the existence of different isoforms and post-translational modifications, some proteins were presented more than once in the two-dimensional electrophoresis gel.
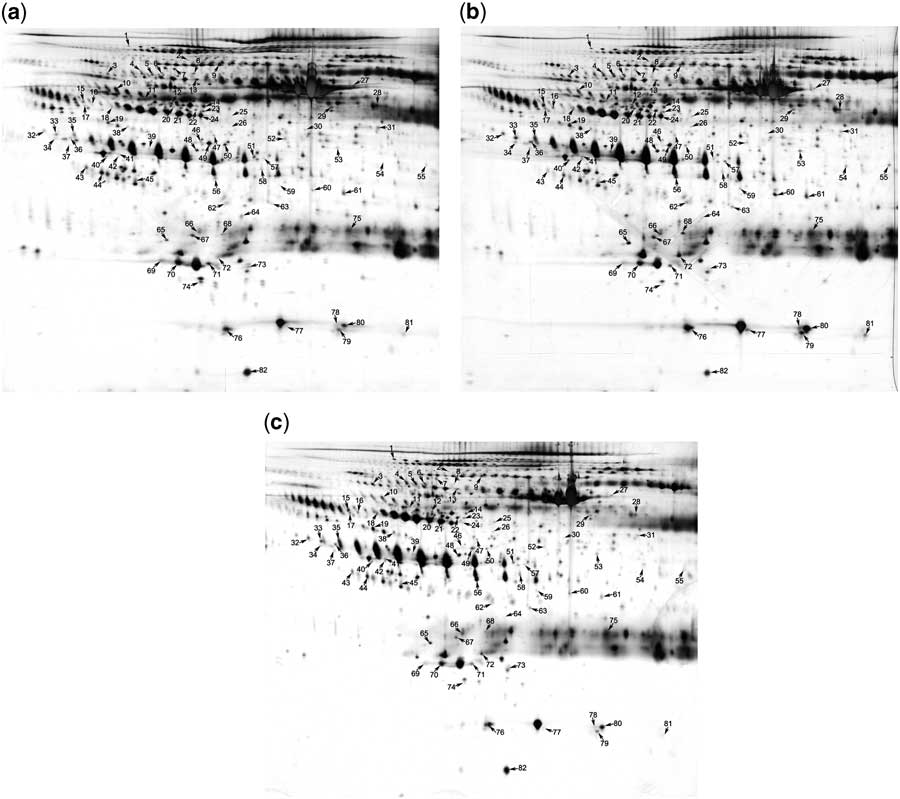
Figure 2 Identification of the differentially expressed proteins using two-dimensional electrophoresis analysis. Proteins from serum exosomes of ( a ) healthy children, ( b ) of patients with Kawasaki disease before intravenous immunoglobulin therapy, and ( c ) of the same patients after intravenous immunoglobulin therapy were subjected to standard two-dimensional electrophoresis gel analysis. Differentially expressed proteins are marked using numbers, which correspond to Supplementary Tables S1 and S2.
Categorisation of the differentially expressed proteins before intravenous immunoglobulin therapy
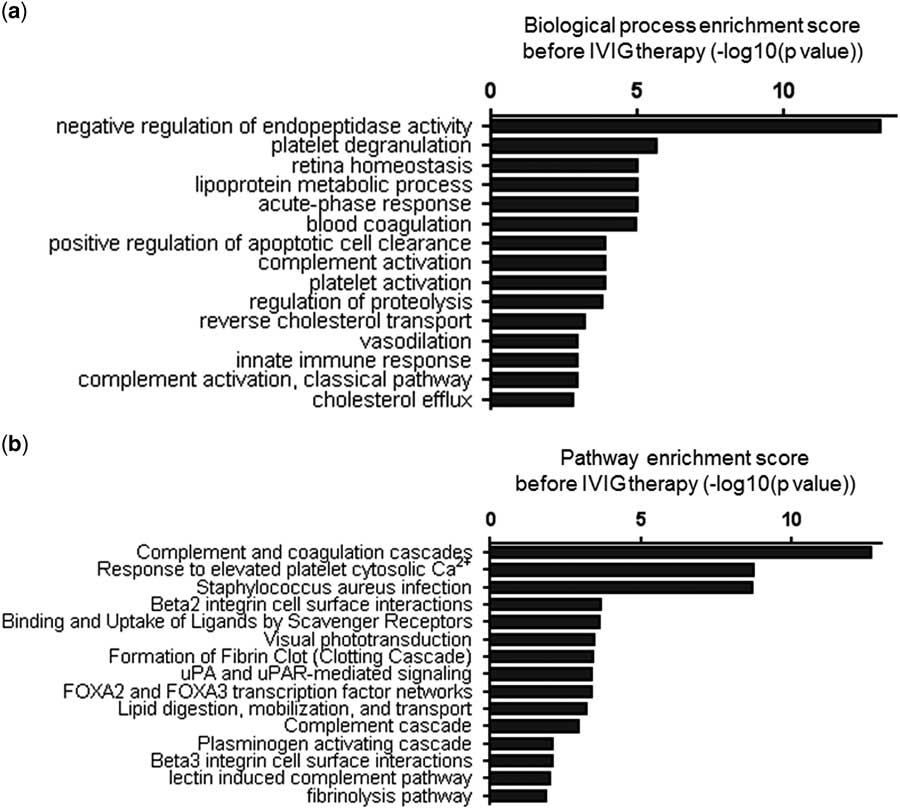
The 69 protein spots of the control group compared with those of patients with Kawasaki disease before intravenous immunoglobulin therapy were grouped on the basis of their gene ontology biological processes using the Cytoscape platform (Fig 3a). The significance of the first 15 annotated functions was ranked according to their p-value. These functions included negative regulation of endopeptidase activity, platelet degranulation, retina homoeostasis, lipoprotein metabolism, acute-phase response, blood coagulation, positive regulation of apoptotic cell clearance, complement activation, platelet activation, regulation of proteolysis, reverse cholesterol transport, vasodilation, innate immune response, complement activation, classical pathway activation, and cholesterol efflux. Most of these functions were associated with blood coagulation and inflammatory and immune responses, relating to the pathological mechanisms of Kawasaki disease.
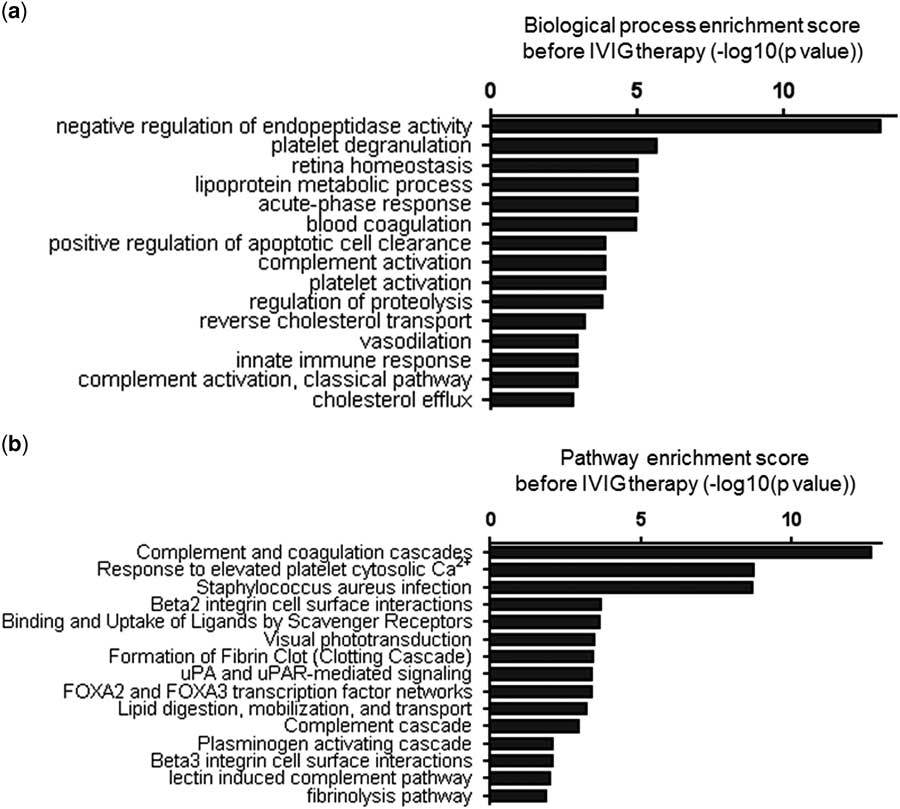
Figure 3 Categorisation of the differentially expressed proteins before intravenous immunoglobulin (IVIG) therapy. ( a ) The top 15 biological processes identified and ( b ) the top 15 pathways analysed by Cytoscape in patients with Kawasaki disease. The log-transformed enrichment scores for each biological process or pathway are indicated on the x-axis.
These differentially expressed proteins were also categorised on the basis of their pathways (Fig 3b). The significance of the first 15 pathways was ranked according to their p-value. These include complement and coagulation cascades, response to elevated platelet cytosolic Ca2+, Staphylococcus aureus infection, β2 integrin cell–surface interactions, binding and uptake of ligands by scavenger receptors, visual phototransduction, fibrin clot formation – the clotting cascade – urokinase-type plasminogen activator and urokinase-type plasminogen activator receptor-mediated signalling, forkhead box A2 and forkhead box A3 transcription factor networks, lipid digestion, mobilisation, and transport. Interestingly, most of these pathways are also related to blood coagulation, inflammation, and immune responses. Collectively, these analyses suggest that the differentially expressed proteins in serum exosomes might reflect the pathological changes in Kawasaki disease.
Categorisation of the differentially expressed proteins after intravenous immunoglobulin therapy
As shown in Figure 4a, the first 15 biological processes that involved the differentially expressed proteins between exosomes of healthy controls and exosomes obtained after intravenous immunoglobulin therapy from patients with Kawasaki disease were listed according to their p-value. The results indicated that the acute-phase response in patients with Kawasaki disease disappeared and the functions of complement activation and innate immune response were enhanced after intravenous immunoglobulin therapy; antibacterial humoral response also emerged. On comparing the pathways associated with the differentially expressed proteins before intravenous immunoglobulin therapy, one pathway for corticosteroids and cardioprotection was identified as being beneficial and helpful in the recovery of patients with Kawasaki disease.
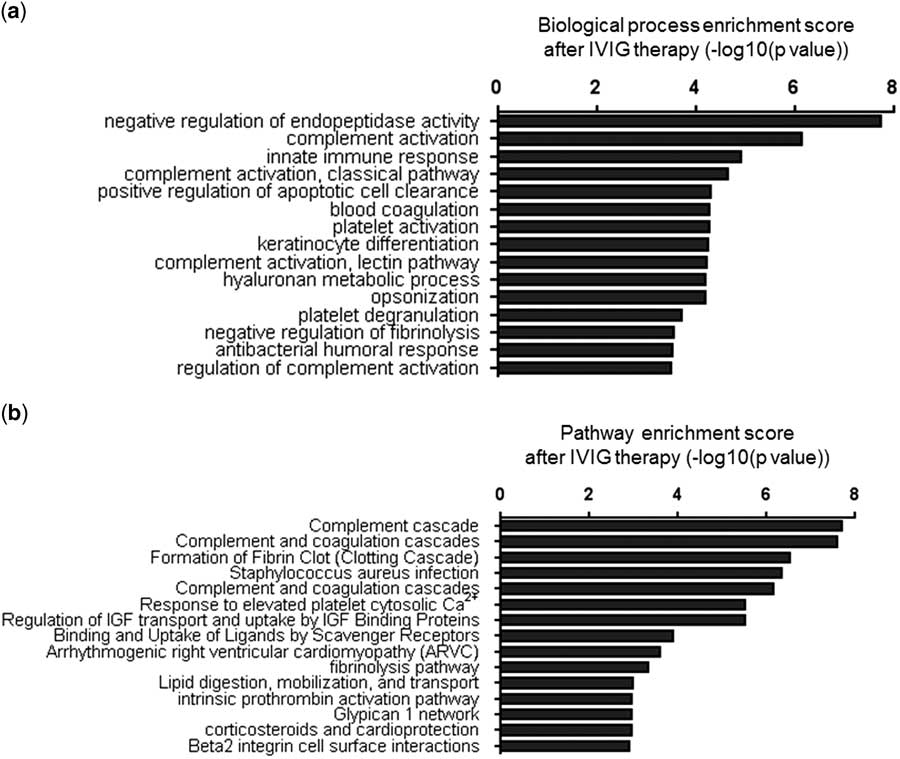
Figure 4 Categorisation of the differentially expressed proteins after intravenous immunoglobulin (IVIG) therapy. ( a ) The top 15 biological processes identified and ( b ) the top 15 pathways analysed by Cytoscape in patients with Kawasaki disease after intravenous immunoglobulin therapy. The log-transformed enrichment scores for each biological process or pathway are indicated on the x-axis.
Interaction network of the differentially expressed proteins
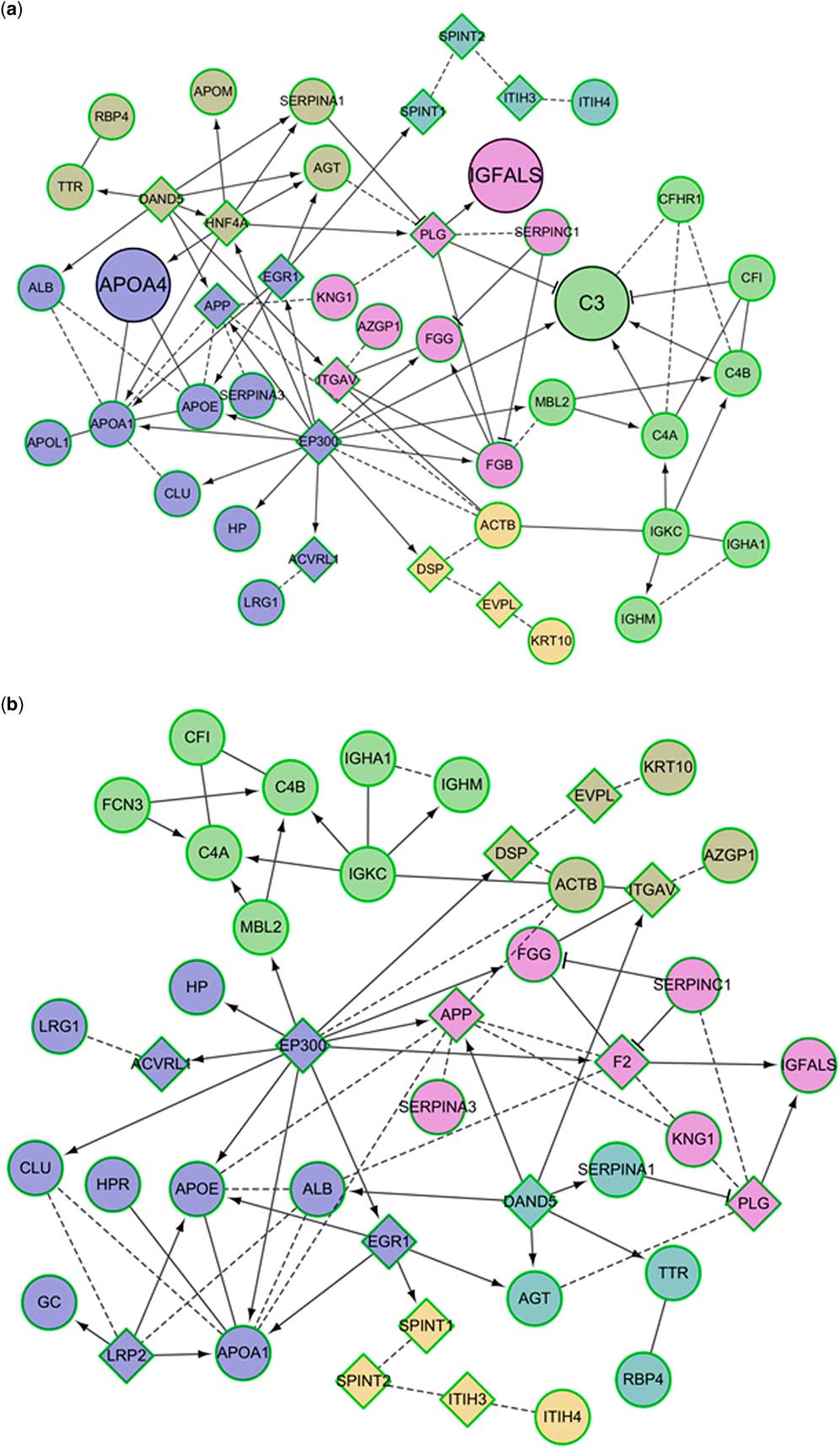
To clarify the relationship between these identified proteins, we used Cytoscape to build an interaction network. As shown in Figure 5, the differentially expressed proteins were associated with each other directly or indirectly. These identified proteins, including those differentially expressed before or after intravenous immunoglobulin therapy, compared with the control, were divided into six different clusters according to their functions, labelled with different colours. The proteins marked with circles were identified by us and those marked with boxes are the linker proteins added by the Cytoscape platform. For differentially expressed proteins before intravenous immunoglobulin therapy, the cluster containing complement C3, complement C4-A, complement C4-B, complement factor H-related protein 1, Complement Factor I protein, mannose-binding protein C, Ig κ chain C region, Ig α-1 chain C region, and Ig μ chain C region is closely related to complement activation and innate immune response, whereas the cluster containing serum albumin, apolipoprotein A-I, apolipoprotein A-IV, apolipoprotein L1, apolipoprotein E, amyloid β-precursor protein, early growth-response 1, α-1-antichymotrypsin, E1A-binding protein p300, clusterin, haptoglobin, activin A receptor-like type 1, and leucine-rich α-2-glycoprotein is closely related to lipoprotein metabolism, reverse cholesterol transport, and cholesterol efflux (Fig 5a). Of these, the complement C3 and apolipoprotein A-IV proteins were not observed in similar clusters obtained for differentially expressed proteins after intravenous immunoglobulin therapy (Fig 5b), suggesting that their expression deviations were corrected after intravenous immunoglobulin therapy.

Figure 5 The interaction networks of the differentially expressed proteins between exosomes from ( a ) patients with Kawasaki disease and healthy controls and ( b ) patients with patients after intravenous immunoglobulin therapy compared with healthy controls. The proteins marked with circles were identified in this study, and those marked with boxes are the linker proteins added by Cytoscape.
Validation of differentially expressed proteins related to intravenous immunoglobulin therapy
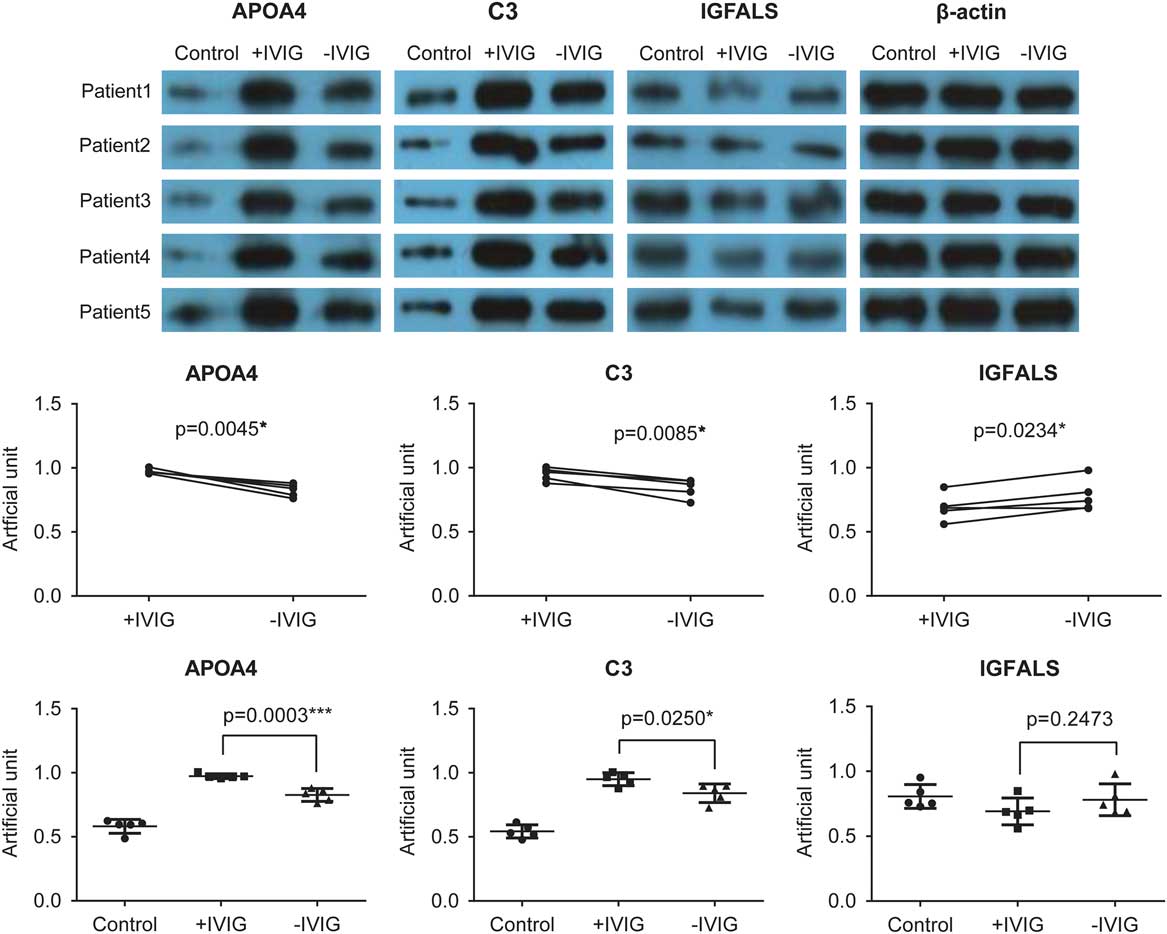
To validate the proteomics results obtained using mass spectrometry, we performed western blot experiments to test the expression of complement C3, apolipoprotein A-IV, and insulin-like growth factor-binding protein complex acid labile subunit. The pre- and post-intravenous immunoglobulin therapy serum exosomes of five patients with Kawasaki disease and five random healthy controls were used (Fig 6, upper panel). The expression of apolipoprotein A-IV and complement C3 was higher before intravenous immunoglobulin therapy in patients with Kawasaki disease than in healthy controls. This increased expression of apolipoprotein A-IV and complement C3 reduced after intravenous immunoglobulin therapy (Fig 6). Conversely, insulin-like growth factor-binding protein complex acid labile subunit expression was lower in patients with Kawasaki disease and was restored to some extent after intravenous immunoglobulin therapy (Fig 6). These results were consistent with those from the proteomics analyses, suggesting that these three proteins are involved in Kawasaki disease.
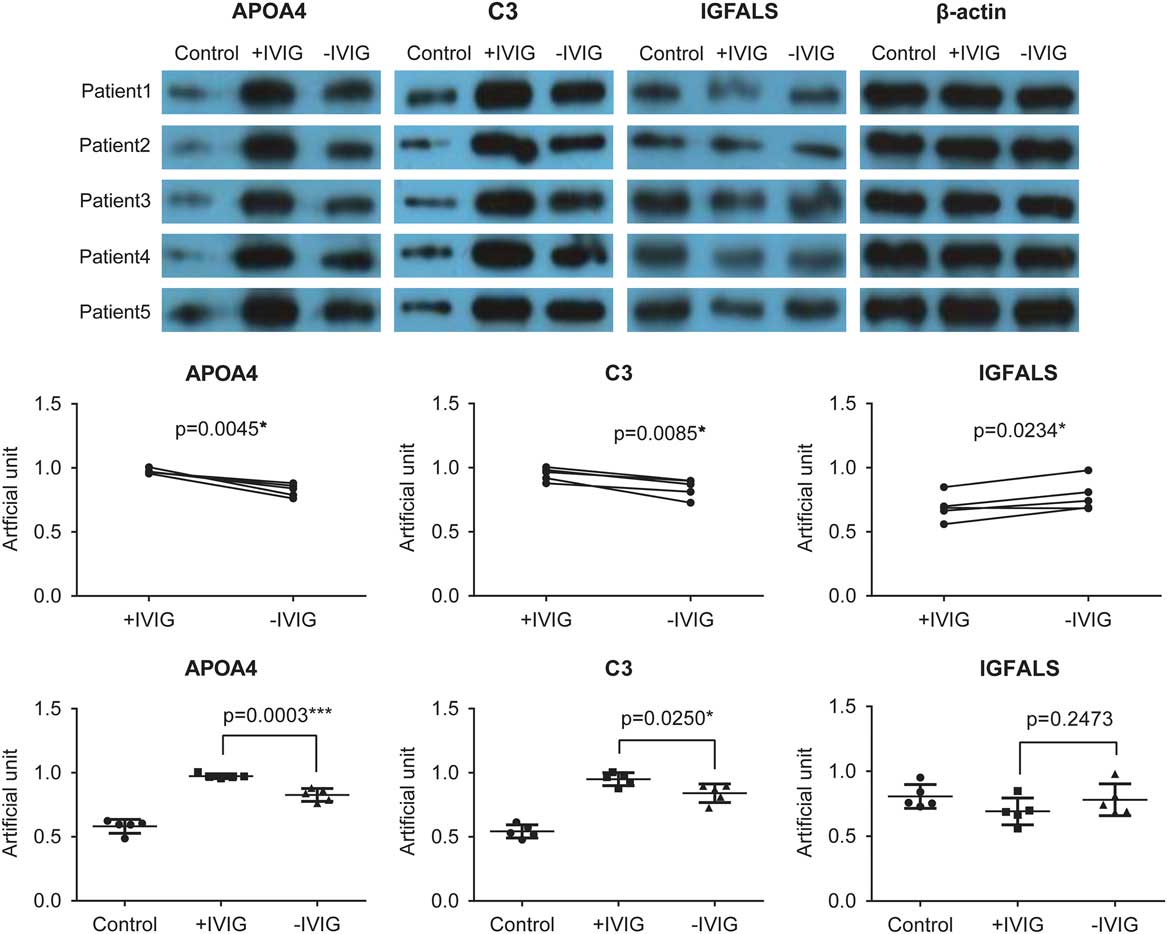
Figure 6 Validation of proteomics results using western blot. In all, five Kawasaki disease patients, other than those recruited for the proteomics analysis, before intravenous immunoglobulin therapy (−IVIG) and after intravenous immunoglobulin therapy (+IVIG) were chosen for the validation experiments. After isolation and lysis of exosomes, the exosomal protein samples were used to perform standard western blot using anti-apolipoprotein A-IV (APOA4), anti-complement C3, and anti-insulin-like growth factor-binding protein complex acid labile subunit (IGFALS) antibodies (upper panel). β-Actin was used as the internal control. The lower panel represents the quantitative analysis of the results shown in the upper panel.
Discussion
Kawasaki disease is characterised by systemic vasculitides accompanied by fever and is mainly observed in infants and young children. If untreated, it often leads to cardiac complications. Intravenous immunoglobulin therapy is usually prescribed to avoid coronary artery dilatation formation in children with Kawasaki disease. Although the underlying mechanism is not clear, intravenous immunoglobulin therapy is known to benefit patients via several immunomodulatory effects involving the complement system, cytokines, and inflammatory cells. One of the mechanisms by which inflammatory cells exert immunomodulation is the secretion of exosomes in the extracellular matrix. Therefore, in this study, we investigated the differentially expressed serum exosomal proteins before and after intravenous immunoglobulin therapy in patients with Kawasaki disease, using two-dimensional electrophoresis and matrix-assisted laser desorption ionisation time of flight/time-of-flight mass spectrometry. Compared with the control group, 69 and 59 differential protein spots were identified in the pre- and post-intravenous immunoglobulin therapy Kawasaki disease groups, respectively. Categorising these differentially expressed proteins showed that they were mostly involved in the biological processes and pathways related to blood coagulation, inflammation, and immune responses, thus being closely associated with the pathological mechanisms of Kawasaki disease. These analyses suggested that the differentially expressed serum exosomal proteins in patients with Kawasaki disease might indicate the pathological changes in Kawasaki disease.
The first proteomics study for Kawasaki disease in 2009 analysed plasma samples from patients with Kawasaki disease and from febrile controls using two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. The differentially expressed proteins displayed increased expression of fibrinogen β and γ chains (FGB and FGG) and α-1-antitrypsin (A1AT/SERPINA1), which is consistent with our observations of the differential proteins in serum exosomes between patients with Kawasaki disease and healthy controls (Supplementary Table S1).Reference Yu, Kuo and Sheen 19
Our results showed that intravenous immunoglobulin therapy reduced acute-phase response in patients with Kawasaki disease and boosted complement activation, innate immune response, and antibacterial humoral response (Figs 3 and 4). In addition, the corticosteroid and cardioprotection pathway that appeared to be activated after intravenous immunoglobulin therapy was beneficial to the patients (Fig 4). We also validated the expression of complement C3, apolipoprotein A-IV, and insulin-like growth factor-binding protein complex acid labile subunit from serum exosomes and found their expression to change in the reverse direction before and after intravenous immunoglobulin therapy (Fig 6), suggesting the involvement of these three proteins in the mechanism underlying intravenous immunoglobulin therapy and their function as potential markers to indicate the effectiveness of intravenous immunoglobulin therapy.
Apolipoprotein A-IV is a protein component of high-density lipoprotein and is synthesised in the intestine. Apolipoprotein A-IV, apolipoprotein A-I, and apolipoprotein E share similar structural features, as they are derived from a common ancestral sequence.Reference Boguski, Elshourbagy, Taylor and Gordon 20 Apolipoprotein A-IV is involved in a broad range of physiological functions such as cholesterol absorptionReference Kalogeris, Rodriguez and Tso 21 and chylomicron assembly.Reference Weinberg, Cook, DeLozier and Shelness 22 In our current study, we found that apolipoprotein A-IV expression was significantly increased in patients with Kawasaki disease, indicating its role in Kawasaki disease pathogenesis. Further, the patients included in our study showed coronary artery dilation, suggesting that increased apolipoprotein A-IV is involved in the pathogenesis of coronary artery dilation. Interestingly, the expression of apolipoprotein A-IV was reduced after intravenous immunoglobulin therapy, which suggests that apolipoprotein A-IV might be involved in the underlying molecular mechanism of intravenous immunoglobulin function. In addition, apolipoprotein A-IV gene polymorphism has been shown to affect cardiovascular risk.Reference Canales, Benedi and Bastida 23 Apolipoprotein A-IV S347 homozygosity was directly associated with increased risk for coronary heart disease and lower plasma apolipoprotein A-IV levels;Reference Wong, Hawe and Li 24 however, further studies are warranted to investigate whether this mutation is involved in the pathogenesis of Kawasaki disease.
The association of the insulin-like growth factor-binding protein complex acid labile subunit with Kawasaki disease has not been mentioned in the currently available literature. Several insulin-like growth factor-binding protein complex acid labile subunit mutations have been identified to be associated with severe insulin-like growth factor 1/insulin-like growth factor-binding protein 3 and acid labile subunit deficiencies, supporting the fact that the insulin-like growth factor-binding protein complex acid labile subunit is involved in the pathogenesis of primary insulin-like growth factor deficiency in children.Reference Fofanova-Gambetti, Hwa and Kirsch 25 The heterozygous insulin-like growth factor-binding protein complex acid labile subunit gene variants might also be related to short stature in children with idiopathic short stature with decreased levels of insulin-like growth factor 1, insulin-like growth factor-binding protein 3, and acid labile subunit.Reference Domené, Scaglia and Martínez 26 In this study, we showed that the decreased expression of insulin-like growth factor-binding protein complex acid labile subunit in patients with Kawasaki disease was restored to some extent after intravenous immunoglobulin therapy (Fig 6), thereby indicating patient response to intravenous immunoglobulin therapy; however, the significance of this restoration needs further investigation.
Conclusions
We analysed the proteomic profile of serum exosomes from patients with Kawasaki disease before and after intravenous immunoglobulin therapy. The biological processes and pathways associated with the differentially expressed proteins uncovered the effects of intravenous immunoglobulin therapy on exosomal proteins and the functional mechanisms underlying intravenous immunoglobulin therapy. Further, a decrease in complement C3 and apolipoprotein A-IV levels or an increase in insulin-like growth factor-binding protein complex acid labile subunit levels after intravenous immunoglobulin therapy might serve as potential indicators of the effective response to intravenous immunoglobulin therapy in patients with Kawasaki disease. These findings provide insights into the mechanism of intravenous immunoglobulin therapy at the level of serum exosomes.
Acknowledgements
None.
Financial Support
This work was funded by the National Natural Science Foundation of China (Project No. 81500275 to H.-L.J.), Natural Science Foundation of Guangdong Province (Project No. 2016A030313080 to H.-L.J.) and Guangzhou City Scientific Research Project Foundation of Technology and Information Bureau (Project No. 201510010287 to L.Z.).
Conflicts of Interest
None.
Ethical Standards
This study was approved by the Ethics Committees at Guangzhou Women and Children’s Medical Center (number [2013]077) and written informed consent was obtained from all guardians.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951117001433