Chylothorax following paediatric cardiac surgery is prevalent (3.8%) and associated with malnutrition, immune compromise, and hypercoagulability and leads to increased hospital length of stay, resource utilisation, and mortality. Reference Panthongviriyakul and Bines1–Reference Czobor, Roth and Prodan8 Although chylothorax was first reported in 1972, aside from isolated local efforts, a standardised approach to management has not been established. Reference Panthongviriyakul and Bines1,Reference Church, Antunez and Dean5,Reference Tutor6,Reference Czobor, Roth and Prodan8–Reference Hermon, Tenner, Burda, Strohmaier, Schlager and Golej18
Lack of standardised care contributes to unnecessary practice variation and precludes the advancement of outcomes research, while protocolised care has been associated with decreased variability and improved outcomes. Reference Winder, Eckhauser, Delgado-Corcoran, Smout, Marietta and Bailly9–Reference Shin, Lee, Park and Park11 Historically, development of multi-site treatment algorithms required large groups of experts to convene over multiple days. Hence, successful multi-disciplinary efforts are rare, difficult, and costly. Adoption of algorithms is further hampered when consensus lacks evidentiary support or when the algorithm is difficult to interpret or apply in a clinical setting at point of care.
To address these widespread and longstanding challenges, we utilised the existing collaborative nature and data-driven infrastructure of the Pediatric Cardiac Critical Care Consortium to form a Chylothorax Work Group. Our initial aim was to develop an algorithm to manage chylothorax following paediatric congenital heart surgery which would include transparent measurements of agreement and understanding of the recommendations developed. Understanding that current chylothorax literature is largely experiential, we anticipate utilising these consensus recommendations as a baseline to guide further iterations in an effort to identify data-driven best practice.
Materials and methods
Patients: The Chylothorax Work Group was formed in October of 2020 with the support of the Pediatric Cardiac Critical Care Consortium Quality Improvement committee. Members of the work group represent 22 centres and consist of more than 60 multi-disciplinary providers: physicians (n = 39), surgeons (n = 2), advanced practice providers (n = 11), dieticians (n = 8), a parent, a quality manager, and a business data analyst (Supplemental Table 1).
Development of algorithm content: The algorithm was developed as a quality improvement initiative. The Key Driver Diagram is shown in Figure 1. Content was derived using existing literature through a PubMed search of chylothorax-related topics in November 2020 and May 2021 and expert opinion through twice-monthly discussions using a virtual platform. Local protocols from 11 participating centres were also reviewed (Supplemental Table 1).

Figure 1. Key driver diagram.
Measuring agreement: Agreement was objectively quantified for 11 central recommendations in the algorithm via an anonymous survey. Response options included strongly disagree, disagree, neutral, agree, and strongly agree, and there was allowance for free-text feedback that was integrated into discussions at subsequent meetings (For survey questions, see Supplemental Table 1). We defined “Consensus” to a recommendation as ≥ 80% of responses reported as “agree” or “strongly agree.” Work group members were encouraged to disseminate the survey to and/or discuss responses with co-workers. When multiple survey responses from a single centre were received, only the lowest degree of agreement from each centre was used to calculate consensus but every response (including free-text comments) was disseminated to the workgroup.
Ex vivo simulations: When algorithms are applied at the bedside, the clinician’s understanding and interpretation may not reflect the intended recommendations of the algorithm developers, leading to unintentional non-compliance. Reference Furlong-Dillard, Miller and Sward19 To determine if the 11 central algorithm recommendations would be correctly interpreted and applied (i.e., understood) at point of use, we developed 11 clinical scenarios (Supplemental Table 2). Algorithm developers and those who did not develop the algorithm were asked to use the algorithm to determine the next recommendation of each clinical scenario. We used these ex vivo simulations to evaluate which recommendations may be difficult to interpret at point of care by those who did not develop the algorithm.
Algorithm definitions: In order to improve clarity within the workgroup and to provide a standard for future work, we established consensus definitions for the terms commonly used when managing chylothorax (Supplemental Table 2).
Results
The algorithm for management of paediatric post-operative chylothorax is shown in Figure 2. The rationale, degree of agreement, and ex vivo simulation results for each of the 11 central recommendations are described below. Degree of agreement reflects responses from 16 institutions, with each institution represented across the 3 surveys. Ex vivo simulation was completed by 17 developers and 33 non-developers. The algorithm is only intended for post-operative patients <18 years of age who are diagnosed with chylothorax within 30 days of cardiothoracic surgery.
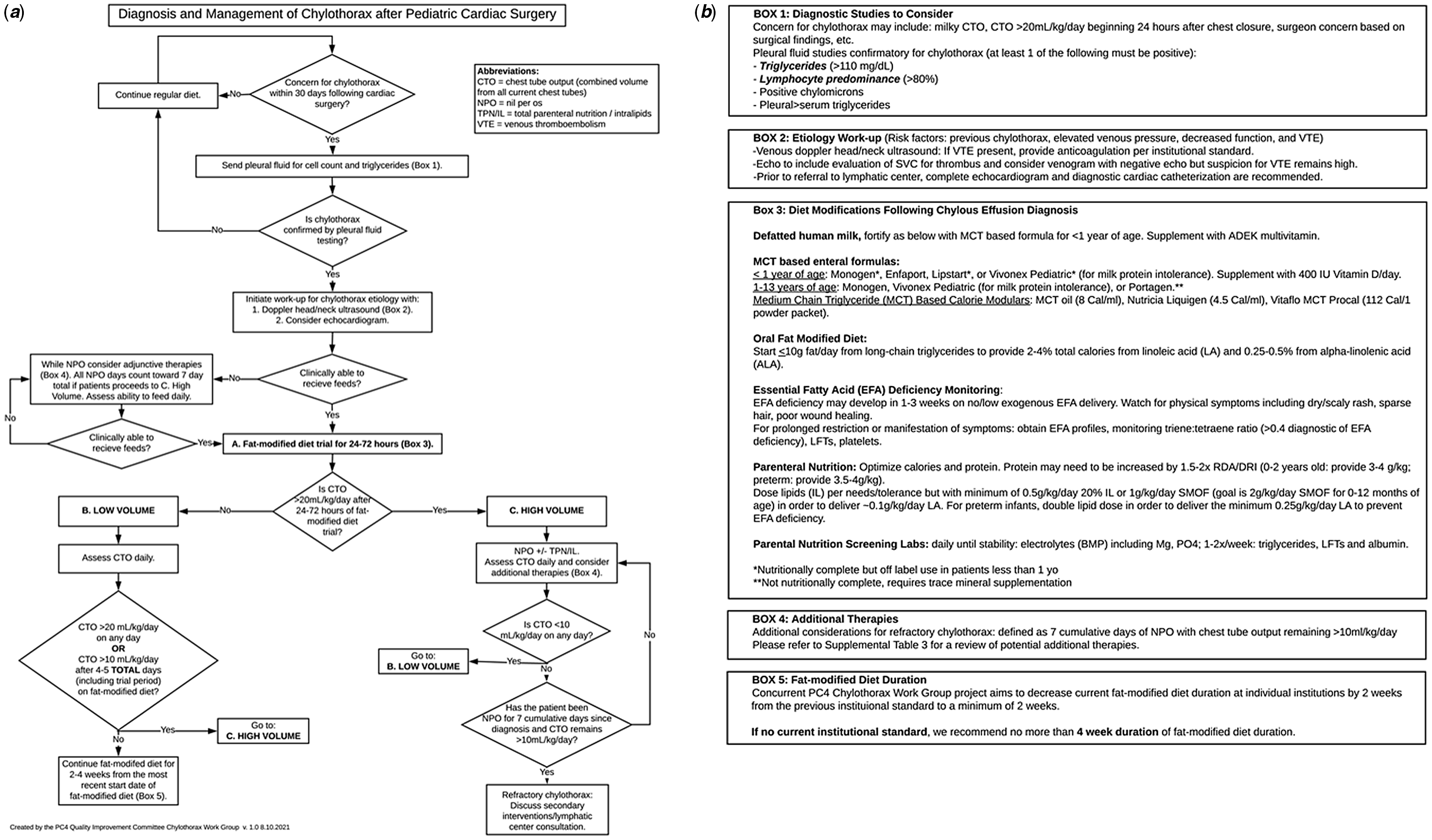
Figure 2. ( a ) Clinical management algorithm for post-operative chylothorax and ( b ) reference boxes for clinical management algorithm.
Recommendation #1: confirm the diagnosis of chylothorax with pleural fluid analysis of cell count and triglycerides
When there is concern for chylothorax within 30 days following cardiothoracic surgery, send pleural fluid for cell count and triglycerides. Pleural fluid testing is diagnostic for chylothorax if triglyceride count is ≥ 110 mg/dL or lymphocyte cell count is ≥ 80%.
Rationale: Presence of chylomicrons when analysed by fluid staining with Sudan III or lipoprotein analysis is considered the “gold standard,” though not widely available. Reference Tutor6,Reference Soto-Martinez and Massie20,Reference McGrath, Blades and Anderson21 Therefore, instead of chylomicron testing we recommend pleural fluid testing for triglycerides and lymphocytes in all patients suspected to have chylothorax. Currently, some centres routinely diagnose chylothorax by clinical appearance of milky fluid aspirated by thoracentesis or appreciated in chest tube output. However, the absence of milky appearance does not exclude the diagnosis of chylothorax, especially in fasting patients, and thus, confirmatory laboratory analysis of pleural fluid is warranted. Reference Panthongviriyakul and Bines1,Reference Schild, Strassburg, Welz and Kalff3,Reference Buttiker, Fanconi and Burger12,Reference Soto-Martinez and Massie20,Reference McGrath, Blades and Anderson21
A recent single-centre report demonstrated that chest tube output is > 15 ml/kg on the day after sternal closure predicts chylothorax with 78% sensitivity and 79% specificity, regardless of output appearance. Reference Winder22 Importantly, testing based on quantity of output alone may lead to earlier diagnosis particularly in the sickest patients where enteral nutrition cannot be provided. Current literature reports that the median time from the operating room to chylothorax diagnosis ranges between 4 and 14 days. Reference Winder, Eckhauser, Delgado-Corcoran, Smout, Marietta and Bailly9–Reference Shin, Lee, Park and Park11,Reference Beghetti, La Scala, Belli, Bugmann, Kalangos and Le Coultre14,Reference Chan, Russell, Williams, Van Arsdell, Coles and McCrindle23,Reference Nath, Savla, Khemani, Nussbaum, Greene and Wells24 Additionally, Chan et al reported an independent association between shorter time to diagnosis and decreased duration of chylothorax drainage. Reference Chan, Russell, Williams, Van Arsdell, Coles and McCrindle23
Agreement: Initially, 75% (12/16) agreement was achieved for pleural fluid testing. Those disagreeing cited the frequency of clinical diagnosis based on milky appearance of pleural fluid. When the algorithm was iterated to reflect optional testing of pleural fluid, follow-up survey agreement dropped to 56% (9/16). Subsequent discussion, highlighting the literature above, resulted in 81% (13/16) agreement to include a recommendation for pleural fluid testing in the algorithm. Reference Panthongviriyakul and Bines1,Reference Schild, Strassburg, Welz and Kalff3,Reference Buttiker, Fanconi and Burger12,Reference Soto-Martinez and Massie20,Reference McGrath, Blades and Anderson21,Reference Chan, Russell, Williams, Van Arsdell, Coles and McCrindle23
Ex vivo simulation: 100% (17/17) of developers and 97% (32/33) of non-developers correctly understood criteria for diagnosing chylothorax. Focused education should be considered at centres where pleural fluid testing is not routinely obtained on every patient suspected to have chylothorax.
Recommendation #2: evaluate the aetiology of chylothorax with vascular ultrasound and echocardiogram
Once diagnosis of chylothorax is confirmed, we recommend vascular ultrasound to evaluate for venous thromboembolism and echocardiogram to evaluate for residual lesions and assess the hemodynamic profile as potential contributing aetiologies.
Rationale: Multiple studies have demonstrated an association between chylothorax and the presence of venous thromboembolism, with incidence ranging from 27 to 52, up to 2.6 times higher than non-chylothorax patients. Reference McCulloch, Conaway, Haizlip, Buck, Bovbjerg and Hoke25,Reference Bauman, Moher, Bruce, Kuhle, Kaur and Massicotte26 Venous hypertension and increased lymphatic transmural pressure have been described as mechanisms for chylothorax, with central venous thrombosis increasing the odds of chylothorax by 6.7 (95% CI, 4.6–9.7). Reference Mery, Moffett and Khan2,Reference Beghetti, La Scala, Belli, Bugmann, Kalangos and Le Coultre14 Therefore, we recommend screening for venous thromboembolism using vascular ultrasound and evaluate for residual lesions and occult hemodynamic disturbances using echocardiogram. When comparing chylothorax aetiologies, Beghetti et al noted that chylothorax secondary to elevated central venous pressure developed significantly later (14 + 2 days post-surgery) than when it was the result of direct injury to the thoracic duct (7.3 + 1 days post-surgery) (p < 0.005). Reference Beghetti, La Scala, Belli, Bugmann, Kalangos and Le Coultre14 Thus, we recommend obtaining the echocardiogram at the time of diagnosis to avoid delay in potential interventions or treatments.
Agreement: 81% (13/16) for vascular ultrasound and 100% (16/16) for echocardiogram.
Ex vivo simulation: 94% (16/17) of developers and 91% (30 of 33) of non-developers correctly understood this recommendation.
Recommendation #3: initiate the management of chylothorax with a fat-modified diet trial
Once diagnosis of chylothorax is confirmed, we recommend initiation of age-appropriate fat-modified diet for 24–72 hours.
Rationale: Medium-chain triglyceride feeds are absorbed directly into the blood stream from the gut and theoretically allow for healing from chylothorax by reducing the flow of chyle through the lymphatic system. Reference Tutor6 Hence, the two most common initial medical management options for chylothorax are either initiation of a fat-modified diet or initiation of nil per os/total parenteral nutrition status in order to decrease production of chyle. Reference Church, Antunez and Dean5,Reference Tutor6,Reference Czobor, Roth and Prodan8–Reference Hermon, Tenner, Burda, Strohmaier, Schlager and Golej18,Reference Hargus, Carson, McGrath, Wolfe and Clarke27
By provision of a 24–72 hour trial of a fat-modified diet in all patients regardless of chest tube output volume, we aim to reduce the potential adverse effects of nil per os and total parenteral nutrition. Reference Calkins, Venick and Devaskar28,Reference Hartman, Shamir, Simchowitz, Lohner, Cai and Decsi29 However, we recognise that those patients who do not develop a significant quantifiable volume of chest tube output after initial chylothorax diagnosis or intervention if thoracentesis is required, may be able to be monitored with their usual diet and not require a fat modification trial. Resolution of chylothorax with fat-modified diet alone (i.e., never made nil per os or requiring a secondary invasive intervention) ranges from 38 to 61%. Reference Shin, Lee, Park and Park11,Reference Buttiker, Fanconi and Burger12 In a single-centre study of 113 patients with post-operative chylothorax, 83% (94/113) trialled a fat-modified diet for 24–36 hours following diagnosis of chylothorax, and 74% (70/94) subsequently resolved without requiring nil per os for high-volume chest tube output. Reference Winder22 Of note, 38% (36/94) of those trialled on a fat-modified diet initially had chest tube output >20 mL/kg/day, but following the trial, 53% (19/36) had a drop in chest tube output to low volume and never required nil per os. This suggests that a fat-modified diet trial is reasonable initial management even in those with high-volume chylothorax.
Because fat is a concentrated source of calories, additional caloric supplementation is required while using a fat-modified diet. Dietary supplementation and monitoring should be performed in coordination with a registered dietician experienced in chylothorax patients. Reference Hill, Peters and Swift30,Reference Anez-Bustillos, Dao and Fell31 Defatted human milk has been shown to be a safe, feasible, and effective alternative to medium-chain triglyceride formulas while providing the known benefits of human milk, though supplementation is still required to achieve appropriate caloric intake (Supplemental Table 2). Reference Kocel, Russell and O’Connor32–Reference Neumann, Springer, Nieschke, Kostelka and Dahnert35 For older children, there is lack of consensus regarding optimal fat restriction with reports ranging from <10 grams/day to <30% of calories from fat per day (Supplemental Table 2). Reference Winder, Eckhauser, Delgado-Corcoran, Smout, Marietta and Bailly9,Reference Sunstrom, Muralidaran and Gerrah36–Reference Marino, Bell, Woodgate and Doolan38 Dietary supplementation and monitoring should be performed in coordination with a registered dietician experienced in chylothorax patients. Reference Hill, Peters and Swift30,Reference Anez-Bustillos, Dao and Fell31
Agreement: 94% (15/16) agreement regarding the initial duration of a fat-modified diet trial, with those in disagreement preferring a longer duration of monitoring prior to stratification.
Ex vivo simulation: 100% (17/17) of developers and 82% (27/33) of non-developers correctly understood this recommendation. To improve compliance to this recommendation, we suggest emphasising the potential impact of a fat-modified diet in decreasing chest tube output to a low-volume status and negating the need for nil per os.
Recommendation #4: if clinically unable to feed, initiate the management of chylothorax with nil per os
If clinically unable to receive a fat-modified at time of diagnosis, we recommend nil per os with total parenteral nutrition supplementation as initial management strategy.
Rationale: Use of total parenteral nutrition to support energy requirements in critically ill children has been established. Reference Mehta and Compher39,Reference Mehta, Skillman and Irving40 However, the utilisation of enteral versus total parenteral nutrition in the setting of chylothorax has not been studied. Intravenous lipid emulsions are delivered directly to the bloodstream and bypass the lymphatic system allowing fat and calorie provision in the setting of chylothorax. Reference Fell, Nandivada, Gura and Puder41
Agreement: 100% agreement regarding provision of total parenteral nutrition if unable to tolerate fat-modified trial.
Ex vivo simulation: Use of nil per os was embedded within multiple clinical scenarios. Understanding was 100% for developers and 73% for and non-developers; see section “management of high-volume chylothorax.”
Recommendation #5: determine high- versus low-volume management arm based on chest tube output of more or less than 20 mL/kg/day
After a 24- to 72-hour trial of fat-modified diet, “high-volume chylothorax” is defined as >20 ml/kg/day of chest tube output, and “low-volume chylothorax” is defined as ≤20 ml/kg/day of chest tube output.
Rationale: We used > 20 ml/kg/day of chest tube output to stratify low versus high-volume chylothorax as this is the cut-off most commonly reported in the literature and utilised by existing single-centre algorithms. Reference Church, Antunez and Dean5,Reference Winder, Eckhauser, Delgado-Corcoran, Smout, Marietta and Bailly9–Reference Shin, Lee, Park and Park11,Reference Hermon, Tenner, Burda, Strohmaier, Schlager and Golej18,Reference Marino, Bell, Woodgate and Doolan38 Lower thresholds are likely to increase nil per os with total parenteral nutrition exposure. Reference Milonakis, Chatzis and Giannopoulos13 Should chylothorax be diagnosed via thoracentesis after initial surgical chest tube removal, we recommend ignoring the “initial dump” when quantifying output in ml/kg/day for stratification. Reference Church, Antunez and Dean5
Agreement: 100% (16/16) agreement for stratification cut-off being >20 ml/kg/day.
Ex vivo simulation: 100% (17/17) of developers and 82% (27/33) of non-developers correctly understood this recommendation. Lack of understanding stemmed from the fact that patients can transition from low- to high-volume management arms at any time and not just during the initial 24–72 hour time period.
Recommendation #6: manage high-volume chylothorax with nil per os
If chest tube output is >20 ml/kg/day after a 24- to 72-hour trial of fat-modified diet, initiate nil per os with total parenteral nutrition and monitor chest tube output daily to determine when the patient is eligible for low output management arm.
Rationale: The success rate of nil per os strategy for patients with high-volume chylothorax ranges between 47 and 73% without the need for invasive intervention. Reference Buckley, Graham and Gaies7,Reference Winder22 Median nil per os duration in the published literature ranges from 3 to 17 days. Reference Winder, Eckhauser, Delgado-Corcoran, Smout, Marietta and Bailly9–Reference Shin, Lee, Park and Park11,Reference Nath, Savla, Khemani, Nussbaum, Greene and Wells24 Additionally, all institutional algorithms reviewed by the Work Group specified nil per os and total parenteral nutrition for high-volume chylothorax (see Supplemental Table 1). The rationale is further supported by studies demonstrating that some medium-chain triglycerides may enter the lymphatic system, especially when medium-chain triglycerides are the primary source of fat, and exacerbate high-volume chylothorax. Reference McCray and Parrish42
Agreement: 94% (15/16) agreement to recommend nil per os and total parenteral nutrition for high-volume chylothorax, with those not in agreement citing the benefits of enteral nutrition and lack of evidence demonstrating that a fat-modified diet is less efficacious than nil per os.
Ex vivo simulation: We simulated understanding of the recommendation for cumulative nil per os duration to be 7 total days before proceeding to refractory management considerations. 100% (17/17) of developers and 73% (24/33) of non-developers correctly understood this recommendation. To improve implementation, we recommend emphasising the importance of minimising nil per os days through early decision-making regarding next steps (e.g., referral to lymphatic centre, thoracic duct ligation) when chylothorax does not resolve using the algorithm.
Recommendation #7: consider refractory management when chylothorax remains high volume after 7 cumulative days of nil per os
If chest tube output is ≥10 ml/kg/day after 7 cumulative days of nil per os, discuss surgical and lymphatic evaluation for refractory chylothorax.
Rationale: Prolonged high-volume chylothorax drainage results in fluid and protein losses which complicate post-operative management and increase risks of haemodynamic deterioration, infection, thrombosis, malnutrition, and poor wound healing. Reference Matsuo, Takahashi, Konishi and Sai16,Reference Mehta and Compher39,Reference Biewer, Zurn and Arnold43,Reference Bender, Murthy and Chamberlain44 Thus, the work group prioritised limiting nil per os/total parenteral nutrition duration given the sparse data supporting its efficacy, balanced with the risks of secondary interventions which are also largely unproven. Two single-centre studies demonstrate that 87% (13/15) and 73% (32/44) of patients initially designated as high-volume chylothorax resolved within 7 days using a nil per os strategy, without requiring further invasive interventions. Reference Winder, Eckhauser, Delgado-Corcoran, Smout, Marietta and Bailly9,Reference Shin, Lee, Park and Park11 Beghetti et al reported that chest tube output >15 ml/kg/day on day 7 of a fat-modified diet was associated with a higher predicted need for surgical intervention (specificity of 80% and sensitivity of 68%). Reference Beghetti, La Scala, Belli, Bugmann, Kalangos and Le Coultre14 Hence, the data suggest that longer nil per os trials are not likely to benefit most patients with protracted chylothorax. Recommendations for managing refractory chylothorax are under development by the workgroup.
Agreement: 94% (15/16) agreement to moving to refractory management options if chest tube output is ≥10 ml/kg/day after 7 days of nil per os.
Ex vivo simulation: 94% (16/17) of developers and 91% (30/33) of non-developers correctly understood this recommendation.
Recommendation #8: transition from high-volume to low-volume management arms based on chest tube output of <10 mL/kg/day
If chest tube output is <10 ml/kg/day at any time, transition to low-volume management arm and resume/begin a fat-modified diet.
Rationale: To decrease nil per os days and return to enteral nutrition as soon as possible, we opted to recommend returning to a fat-modified diet based on chest tube output volume, rather than a specified number of days. Since adopting this approach in 2017, Winder et al have treated 44 patients with high-volume chylothorax by transitioning to fat-modified diet anytime chest tube output is <10 ml/kg/day, with only 2 subsequently needing to resume nil per os based on increased chest tube output. Reference Winder, Eckhauser, Delgado-Corcoran, Smout, Marietta and Bailly9,Reference Winder22 Both of those patients had undergone the Glenn operation and had late-presenting refractory chylothorax. After implementation of a clinical practice guideline that similarly encouraged resuming fat-modified diet anytime chest tube output decreased to <10 ml/kg/day, Yeh et al demonstrated significant reductions in total duration of nil per os days, the number of times a patient was made nil per os, and total ICU and hospital lengths of stay. Reference Yeh, Brown and Kellogg10
Agreement: 75% (12/16) initial agreement. Those in disagreement advocated for a longer duration of chest tube output <10 ml/kg/day before re-initiating a fat-modified diet as prescribed in the low-volume arm. Discussing the experiences described above with the work group resulted in 88% (14/16) agreement.
Ex vivo simulation: 94% (16/17) of developers and 88% (29/33) of non-developers correctly understood this recommendation. To improve implementation, we recommend daily discussions regarding eligibility to transition from high-volume to low-volume management arms.
Recommendation #9: manage low-volume chylothorax with a fat-modified diet
Continue a fat-modified diet for 4–5 days if the chest tube output remains ≤20 ml/kg/day after the initial 24- to 72-hour fat-modified diet trial period or once the chest tube output is <10 ml/kg/day at any time in high-volume patients.
Rationale: A positive response to a fat-modified diet is reported in 74%–90% of patients. Reference Church, Antunez and Dean5,Reference Shin, Lee, Park and Park11,Reference Milonakis, Chatzis and Giannopoulos13–Reference Densupsoontorn, Jirapinyo and Wongarn15 The rationale for utilising a fat-modified diet in the management of chylothorax is described above.
Agreement: 100% (18/18) agreement in defining responsiveness to low-volume management as < 10 ml/kg/day of chest tube output after 4–5 days of fat-modified diet.
Ex vivo simulation: 94% (16/17) of developers and 97% (32/33) of non-developers correctly understood this recommendation.
Recommendation #10: transition from low-volume to high-volume management arms based on persistent or high chest tube output
If chest tube output is >10 to ≤20 ml/kg/day after 4–5 total days of fat-modified diet or >20 ml/kg/day at any time, transition to the high-volume management arm.
Rationale: The transition from the low-volume to high-volume management arm is anticipated to be a rare but important type of patient to delineate. At Primary Children’s Hospital, only 6% (4 of 69) of patients who initially entered a low-volume management arm subsequently transitioned to a high-volume management arm. Of these 4 patients, 2 received secondary interventions at 16 and 26 days of chylothorax. Existing published protocols that allow for transition between low- and high-volume management arms are associated with decreased duration of nil per os days, median chest tube days, median hospital and ICU lengths of stay, and reductions in need for pleurodesis or thoracic duct ligation. Reference Winder, Eckhauser, Delgado-Corcoran, Smout, Marietta and Bailly9–Reference Shin, Lee, Park and Park11
Agreement: 100% (16/16) agreement for transitioning to the high-volume management arm when chest tube output is >20 ml/kg/day any time. Agreement for transitioning to the high-volume arm if chest tube output is ≥10 ml/kg/day after 4–5 days of fat-modified diet was 94% (15/16).
Ex vivo simulation: 94% (16/17) of developers and 91% (30/33) of non-developers correctly understood this recommendation.
Recommendation #11: fat-modified diet duration of 2–4 weeks
Continue fat-modified diet for a minimum of 2 weeks to a maximum of 4 weeks from the time a diet is started/resumed after any nil per os days.
Rationale: A 6-week fat-modified diet is the most commonly reported duration in current literature. Reference Yeh, Brown and Kellogg10,Reference Milonakis, Chatzis and Giannopoulos13,Reference Winder22,Reference Marino, Bell, Woodgate and Doolan38 However, success has been demonstrated with 17 days of fat-modified diet. Reference Bender, Murthy and Chamberlain44 Similarly, Winder et al have reported a consecutive cohort of 66 patients with post-operative chylothorax successfully treated with 2 weeks of fat-modified diet without any recurrence of chylothorax within 30 days of resuming a regular diet. Reference Winder, Eckhauser, Delgado-Corcoran, Smout, Marietta and Bailly9,Reference Winder, Vijayarajah and Reeder45 This cohort was inclusive of all chylothorax patients, with the exception of those who underwent Glenn or Fontan operations. 48% (n = 32) had a Society of Thoracic Surgeons-European Association for Cardiothoracic Surgery category of 4 or 5, 53% (n = 35) required nil per os for high-volume chylothorax, and 44% (n = 29) had chest tubes for 10 or more days. A multi-centre quality improvement project to decrease duration of fat-modified diet from the current baseline down to two weeks is currently underway within the Chylothorax Work Group.
Agreement: 100% (16/16) agreement to a duration of 2–4 weeks of a fat-modified diet following final achievement of low-volume criteria. The work group felt it was appropriate to recommend this range given the successful experience at Primary Children’s with shorter fat-modified diet durations, while still incorporating existing algorithmic approaches.
Ex vivo simulation: 100% (17/17) of developers and 100% (33/33) of non-developers correctly understood this recommendation. To improve implementation, we recommend discussing standardising institutional fat-modified diet duration and decreasing as able to the shortest duration of comfort.
Ancillary therapies
Despite the widespread use of ancillary therapies, review of the literature does not strongly support the use of any existing options. See Supplemental Table 3, “Literature review of ancillary medical therapies used to treat chylothorax,” for more detail.
Discussion
The algorithm developed represents the first multi-centre effort to standardise the diagnosis and management of post-operative chylothorax in children. Content was derived from a comprehensive multi-disciplinary review of the literature, expert consensus, and review of 11 existing chylothorax treatment protocols.
Novel to this algorithm is a transparently reported measurement of the degree of agreement for each recommendation in the algorithm. Additionally, the algorithm recommendations have undergone ex vivo simulations using clinically relevant scenarios to determine if recommendations are understood according to the developers’ intentions. Not surprisingly, understanding was higher in developers than non-developers (average correct answers to ex vivo simulation questions 97 versus 90%), but overall simulation demonstrated a high level of understanding in both groups. Additionally, using these unique methods, an improved a priori insight is obtained about potential areas of disagreement and aspects of the guidelines that may be difficult to interpret or implement in a clinical setting. Another unique aspect of this process was that the algorithm was entirely developed via a virtual platform during a global pandemic. Nonetheless, an average of 21 people attended each of the 12 work meetings and included a robust multi-disciplinary group (Supplemental Table 1).
Through the establishment of this multi-institutional Chylothorax Work Group, we are able to utilise quality methods to standardise care, measure outcomes, and iterate on our proposed guidelines. For example, we have clarified and standardised the diagnosis of chylothorax by recommending sending for pleural fluid studies regardless of fluid appearance, thereby moving away from unnecessary practice variation that currently exists in identifying chylothorax patients. We have also proposed standard definitions for the important features of chylothorax such as “high” versus “low volume” and “refractory,” which are important in understanding the association of these clinical milestones on patient outcomes across multiple centres. Overall, the guidelines begin to standardise clinical practice, which is necessary to advance research and benchmark outcomes.
In tandem with guideline development, we also began to obtain Institutional Review Board approval and data use agreements across centres. Data collection is underway in participating centres to better understand patient characteristics and daily nutritional and medical management. We hope to direct future iterations of the guidelines regarding important, but under-researched topics, such as nutritional management, the effect of time to chylothorax diagnosis on chest tube duration, and the impact of nil per os on future feeding tolerance. Eventually, we hope to provide a multi-institutional perspective of chylothorax management and related outcomes.
Limitations to the guidelines include the reliance on single-centre protocols and expert experience due to a lack of prospective trials to guide management of chylothorax. Therefore, deep-rooted, anecdotal practices may have been incorporated into the guidelines. We recognise that publication of these guidelines may unintentionally result in widespread adoption of the guidelines as best practice, when the intent is to utilise the guidelines as a standardised platform to study chylothorax and move toward identifying best practice. We limited the recommendations to the first week of medical management and first-line nutritional therapies only, given the paucity of data to support any ancillary medical treatments and variation in surgical approach when intervention for chylothorax may be warranted.
Future efforts within the Chylothorax Work Group are to address chylothorax-related venous thromboembolism prevention, earlier detection, and refractory chylothorax. Efforts are underway to determine “best practices” related to diagnostics and treatments in patients who may ultimately be managed by lymphatic referral centres including which interventions/studies should be avoided or considered prior to referral.
Conclusions
We created a multi-centre chylothorax management algorithm by achieving consensus using surveys to determine agreement to algorithm recommendations and ex vivo simulation to test user understanding of recommendations. We anticipate future iterations to refine the management of chylothorax as we seek to further understand aetiologies, comorbidities, and effective treatment strategies for this difficult post-operative complication.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122001871.
Acknowledgements
We wish to acknowledge the efforts of every participating member of the Chylothorax Work Group for their time, energy, and enthusiasm dedicated to the creation of these guidelines and ongoing chylothorax research. We wish to thank the Pediatric Cardiac Critical Care Consortium Quality Improvement Committee for their support throughout this endeavour.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.





