Published online by Cambridge University Press: 18 April 2005
During the last two decades, several advances have resulted in marked improvement in medium-term survival, with excellent quality of life, in children undergoing cardiac transplantation. Improved outcomes reflect better selection of donors and recipients, increased surgical experience in transplantation for complex congenital heart disease, development of effective surveillance for rejection, and wider choice of immunosuppressive medications. Despite all of these advances, recipients continue to suffer from the adverse effects of non-specific immunosupression, including infections, induction of lymphoproliferative disorders and other malignancies, renal dysfunction, and other important end-organ toxicities. Furthermore, newer immunosuppressive regimes, thus far, appear to have had relatively little impact on the incidence of chronic rejection. Progress in our understanding of the immunologic mechanisms of rejection and graft acceptance should lead to more targeted immunosuppressive therapy and avoidance of non-specific immunosupression. The ultimate goal is to induce a state of tolerance, wherein the recipient will accept the allograft indefinitely, without the need for long-term immunusupression, and yet remain immuno-competent to all non-donor antigens. This quest is currently being realized in many animal models of solid organ transplantation, and offers great hope for the future.
Kantrowitz and associates,1 in December, 1967, were the first to perform cardiac transplantation in a child. Although this infant survived only a few hours after transplantation, the procedure created history, and demonstrated the technical feasibility of cardiac transplantation in children. Interest in transplantation of the heart in children, and cardiac transplantation in general, declined throughout the 1970s, due to high mortality resulting primarily from lack of effective immunosuppressive medications. Over the next two and half decades, great progress has been made in clinical cardiac transplantation. Most important of all was the discovery and introduction of cyclosporine in the late 1970s and early 1980s. This resulted in dramatic improvement in survival of transplanted adults, and led to renewed interest in transplantation of the heart in children. Other advances throughout the 1970s and 1980s included improved selection of candidates and donors, enhanced preservation of the donor heart, refinements in surgical technique, the development of endomyocardial biopsy for surveillance of rejection, and the histopathologic description and standardization of allograft rejection. The Stanford program for children commenced in the 1970s in the pre-cyclosporine era, and the program in Pittsburgh began in 1982. In this review, I will summarize some of the many lessons learned over the last 20 years, discuss ongoing controversies, highlight recent advances in our knowledge, and describe some of the exciting prospects for the future of clinical cardiac transplantation in children.
Transplantation of the heart is generally considered indicated when expected survival is less than 1 or 2 years, and/or when there is unacceptable quality of life secondary to irreparable cardiac disease. Dilated cardiomyopathy, and complex congenital heart defects, remain the primary indications, and together account for over nine-tenths of transplantations undertaken in children.2 Other conditions, such as restrictive and hypertrophic cardiomyopathies, primary cardiac tumors, and re-transplantations, account for less than one-tenth of the patients listed for transplantation (Fig. 1). The appropriate indications for transplantation in childhood were the focus of a recent report from the Pediatric Committee of the American Society of Transplantation.3 This consensus statement also outlined several areas of continuing controversy. A number of these are worthy of further consideration.

Figure 1. Indications for heart transplantation among 169 recipients, undergoing 180 transplantations, at Children's Hospital of Pittsburgh over the period 1982–2002.
Perhaps the most controversial indication is for the newborn with unpalliated hypopolastic left heart syndrome. Survival rates in excess of 80% at one year may be achieved in experienced centers with primary transplantation for this condition.4 Similar results may be achieved with staged reconstruction, the so-called Norwood approach.5 “Head-to-head” comparisons of these therapies should ideally involve:
Most centers have moved away from transplantation, and towards staged reconstruction, for this group of neonates, partly in light of the high mortality prior to transplantation, which some have reported to approach 30%.6 In fact, only approximately 70 transplants are performed in infants each year in the United States of America. It has been estimated that only approximately 5% of infants with hypoplastic left heart syndrome or related disorders could be palliated with transplantation due to the scarcity of donor organs. A pragmatic approach would involve identification of patients with adverse risk factors for successful staged palliation, such as a miniscule aorta, or severe right ventricular dysfunction, and to reserve transplantation for this group. Of course, infants with certain anatomic variants, such as intact interatrial septum, represent poor candidates for either staged repair or primary transplantation.7
Survivors of childhood cancer previously treated with anthracyclines, with or without mediastinal radiation, represent a challenging group of patients. The period of time that a child should be free of disease prior to transplantation should be determined in collaboration with a pediatric oncologist, and will be dependent on the type of tumor, its stage, and the response of the disease. The risk of recurrent disease, or new onset malignancy, is poorly defined. In a recent review of 17 cases from 3 centers, only one case of recurrence was identified after transplantation, and no new malignancies were encountered.8 Much less experience exists with concurrent, or recently treated, malignancy,9 especially primary cardiac tumors. Our only experience in this regard is with one patient with primary angiosarcoma of the heart, who was treated with chemotherapy followed by transplantation. This case was associated with early recurrence.10
Transplantation of children with chronic infection with either hepatitis B or C, or human immunodeficiency virus, remains controversial. Indeed, there is very little experience of transplantation in children in any of these areas, and information is generally extrapolated from data with adults, and from transplantation of other organs. Small trials of liver transplantation in patients with human immunodeficiency virus infection are underway. While most centers consider infection with this virus a contraindication to transplantation, decisions must ultimately be evidence-based rather than reflecting anecdotes, personal bias, or “gestalt”. Most importantly, we must predefine what we consider an acceptable outcome. If we believe, for example, that candidates should have an estimated one year survival of >70%, then we would not be justified in performing heart transplantation for infants and children receiving support with extracorporeal membrane oxygenation. For candidates with hepatitis B and C infection, it is important to evaluate the extent of any active hepatitis and cirrhosis. Several reports have suggested progression, or development, of chronic liver disease in many recipients having hepatitis B and C following transplantation.11–14 The “natural” history of these infections in the population of children undergoing transplantation warrants study through multi-centric collaborations.
Improvements in outcome over the last 20 years reflect reductions in perioperative mortality, as well as reduction in deaths beyond initial discharge from hospital.15 These improvements do not reflect a reduced acuity of the patients coming to transplantation. Indeed, most recipients are now hospitalized at the time of transplantation. Many undergo transplantation while receiving artificial ventilation, and about 10% of our own recipients were receiving mechanical circulatory support at the time of transplantation.
Children with complex congenital heart disease now represent approximately half of all referrals for consideration for transplantation. Other than the newborn with hypoplastic left heart syndrome, most have undergone multiple prior surgical palliative or corrective procedures.16 Frequently, there are stenoses, hypoplastic segments, or even discontinuities of the branch pulmonary arteries. Anomalies of systemic and pulmonary venous return, particularly in the context of isomerism or mirror imaged atrial arrangement, pose additional challenges for the surgeon. A series of reports have described specialized surgical techniques for dealing with these complex anomalies of the heart and great vessels, including abnormalities of atrial arrangement.16–20 In experienced centers, survival has been accomplished comparable to patients with cardiomyopathy, though the donor ischemic times, post-operative stays in intensive care, and length of hospitalization are prolonged compared to patients transplanted for cardiomyopathy.16, 17 Failure to recognize the presence of significant aorto-pulmonary collateral circulation in patients with cyanotic heart disease may contribute to high output failure occurring soon after transplantation.21 This emphasizes the importance of comprehensive preoperative anatomic evaluation and planning in this difficult group of patients.
The patient with a “failed Fontan” procedure poses additional challenges related to premorbid states such as protein losing enteropathy, chronic liver disease, and pulmonary arteriovenous malformations. The latter are most common in those with a previous classical Glenn anastomosis, and when hepatic venous return is excluded from the cavo-pulmonary repair, especially in the setting of left isomerism. Resolution of small pulmonary arterio-venous malformations may occur after transplantation,22 though it would seem unlikely that large lesions will regress rapidly. In the latter setting, the patient may remain severely cyanotic after transplantation, potentially leading to primary failure of the transplanted heart from hypoxemia. It should also be recognized that increasing numbers of patients with complex palliated congenital heart disease are being referred for consideration of transplantation in adult life. It is important to note that we cannot readily extrapolate results from children to adults. In general, the results of transplantation for congenital heart disease in adults are poor compared to those in children, though the precise reasons for this are not clear.23, 24
Specialized techniques for implantation are required for complex congenital defects, as outlined above. Where anatomy is straightforward, two basic techniques may be utilized. For many years, most orthotopic transplantations were performed using biatrial anastomoses, based on the original technique of Lower and Shumway.25 This technique avoids individual systemic and pulmonary venous anastomoses. The recipient is left with capacious atrial chambers, comprising donor and recipient components, which contract asynchronously. It has been suggested that atrial contribution to cardiac output may be superior with near to total cardiac transplantation.26 A small cuff of left atrial tissue is left in place, incorporating all pulmonary veins, and the entire right atrium is removed. Bicaval anastomoses are then performed. This technique results in more normal anatomical result. It has been suggested that it improves sinus nodal function, invokes less tricuspid regurgitation, and improves exercise performance.27 While we routinely utilize this technique, there is only preliminary data in children to show that outcomes are improved.28
A key issue in the evaluation of any candidate for transplantation is pulmonary vascular resistance. If this is excessive and fixed, the ischemic, thin walled right ventricle of the transplanted heart will fail acutely, and it will not be possible to wean the patient from cardiopulmonary bypass. The upper-limit of indexed pulmonary resistance that is acceptable remains a subject of debate. In adults, pulmonary vascular resistance >5 units, and transpulmonary gradients >15 mmHg, are associated with increased perioperative mortality, and are generally considered contraindications to orthotopic transplantation.29 Children appear able to undergo successful transplantation with higher indexed resistances than their adult counterparts.30 Using guidelines similar to those proposed by Addonizio,31 we consider children with indexed pulmonary vascular resistance <10 units to be acceptable candidates, though those in the range of 6 to 10 units are considered at increased risk of acute right heart failure. Selected patients with indexed pulmonary resistance >10 units are also accepted for transplantation if a significant drop in resistance to below 10 units can be achieved with vasodilator testing. In general, we evaluate hemodynamics with general anesthesia to allow for hyperventilation, administration of 100% oxygen, and administration of nitric oxide at up to 80 parts per million. We have found that the addition of intravenous vasodilators rarely produces further immediate fall in pulmonary vascular resistance in the catheterization laboratory. If the latter remains borderline, we admit the patient to hospital and treat for 1 to 2 weeks with intravenous dobutamine and milrinone, and then restudy. Impressive falls in pulmonary vascular resistance have been achieved using this strategy. Over the last 15 years, approximately one-fifth of our candidates have been transplanted with indexed pulmonary vascular resistance >6 units. Only one death due to right heart failure has occurred, and in that instance we ignored our own guidelines, in that a baseline resistance of 14.6 indexed units fell to only 12 with vasodilator therapy.
Mechanical circulatory support has been utilized to bridge infants and children to cardiac transplantation for many years.32 Extracorporeal membrane oxygenation has been most widely used in infants and small children, since ventricular assist devices are generally not available for use in this population. In general, it is hard to support children more than two weeks using this technology, due to escalating problems with bleeding, sepsis, and secondary end organ dysfunction. Pre-transplant mortality is high, and the postoperative course is often difficult. The overall chance of achieving hospital discharge after listing for transplantation is around 50%.32 A recent report suggests that persistent renal failure while on extracorporeal membrane oxygenation support is a major adverse prognostic factor for survival in children being bridged to transplantation.33 This should represent a relative, if not absolute, contraindication to proceeding with transplantation.
Older children can be successfully bridged to transplantation using ventricular assist devices originally designed for use in adults.34, 35 We have successfully bridged eight children to transplant using left heart or biventricular support with Thoratec (Thoratec Laboratories, Berkeley, CA) and Novacor (Novacor Division-World Heart Coorporation, Oakland, CA) assist devices. The Thoratec system has been successfully employed in children <20 kg. In Europe, more effort has been extended to produce pneumatically driven paracorporeal devices for children. The greatest experience is with the pediatric version of the “Berlin Heart” (Mediport Kardiotechnik, Berlin, Germany). A second, similar, system has also been developed in Germany (Medos HIA VAD, Stolberg, Germany). Both systems have been used successfully to bridge small children, including neonates, to transplantation.36, 37 The major stumbling blocks to further development of such devices for children are the small market, prolonged time to develop and test new systems, and the very high costs of such development.
The recipient of a transplanted heart will invariably mount an immune response to foreign antigens contained within the graft. Thus, all recipients, even the neonate, require potent immunosuppressive therapy. This immune response is most vigorous in the early weeks and months after transplantation, but is generally believed to persist for the life of the graft. Unlike the case of some recipients of transplanted livers, there is no evidence that recipients of cardiac transplantation can be withdrawn from immunosuppressive therapy, even late after the transplantation. Clinical immunosuppression aims to prevent or minimize the immune response of the host to donor antigens, while avoiding complications of iatrogenic immunodeficiency such as infections and malignancy. It is also important to minimize non-immune toxicities, such as diabetogenic effects, nephro- and neurotoxicity, hyperlipidemia, suppression of the bone marrow, and cushingoid side effects. Ultimately, the goal is to avoid long-term immunosuppressive drugs altogether by inducing a state of donor-specific tolerance. In this section, I will deal with both immune and non-immune complications of cardiac transplantation.
Acute failure of the transplanted heart is the most common cause of death in the first 30 days after transplantation and occurs with greater frequency in infant recipients.2 From 1 to 5 years after transplantation, acute cellular rejection and infection are the commonest causes of death. Beyond 5 years, chronic rejection becomes the dominant cause of loss of either the heart or the patient. Understanding the timing of, and risk factors for, acute rejection are vital for designing logical strategies for surveillance and prevention. It must also be noted that there is an important distinction between acute rejection and acute dysfunction of the heart. The two terms are not synonymous. Most acute rejection in the heart is not associated with overt dysfunction, though subtle subclinical abnormalities of diastolic function are common. When cardiac failure does occur in the first few years after transplantation, acute cellular rejection is the most likely diagnosis, though other pathologies must be entertained. These include humoral, antibody mediated, rejection,38 acute presentation of chronic rejection due to post-transplantation coronary arterial disease, and even viral myocarditis. There then remains a mysterious group of patients in whom acute dysfunction, sometimes reversible with augmentation of immunosuppression, occurs without evidence of significant cellular, humoral, or chronic rejection.39 The pathophysiology of this phenomenon remains an enigma, making it hard to develop logical strategies for treatment.
The majority of recipients in childhood will experience at least one episode of moderate or severe acute cellular rejection. Data from the Pediatric Heart Transplant Study, a research study group of 23 North American centers undertaking transplantation in children, shows that approximately two-thirds of recipients are free from rejection by 1 month after transplantation, but this has fallen to under one-third by 1 year (Fig. 2). The peak hazard, or instantaneous risk, for rejection is around 1–2 months after transplantation. Late episodes of acute rejection, that is after more than 1 year after transplantation, have also recently been studied within the consortium.40 Episodes of acute rejection occurring late after transplantation were diagnosed in one-quarter of recipients, with a probability of freedom from late rejection of 82% at 2 years, and 73% at 3 years after transplantation. In contrast to the first episodes of rejection occurring early after transplantation, the hazard for late rejection is more constant, with an ongoing risk for as long as the cohort has been followed. This ongoing risk for acute rejection correlates with the clinical observation that recipients do not tolerate discontinuation of immunosuppression, which at this time must be considered a life long therapy.

Figure 2. Probability of freedom from acute rejection among 1114 primary transplantations recorded in the files of the Pediatric Heart Transplant Study, from 1993 to 2000. The material is used with permission of the Pediatric Heart Transplant Study.
Several recent analyses from the Pediatric Heart Transplant Study sought the risk factors for early acute rejection, late acute rejection, and rejection with hemodynamic compromise.40–42 Older age at transplantation was the strongest predictor of risk for first rejection, and risk for an increased number of episodes of rejection within the first 6 months after transplantation.41 These data are consistent with other observations that suggest that young infants may be less prone to acute rejection.43 Risk factors for the initial episode of rejection when occurring more than one year after transplantation were greater than one episode of rejection in the first year after transplantation, black race of the recipient, and older age at transplantation.40 Risk factors for rejection with severe hemodynamic compromise, a serious complication with high mortality, were older age at transplantation and black race of the recipient.42
Recently, there has been significant interest in the investigation of genetic risk factors of the recipient for outcomes regarding both the transplanted organ and the wellbeing of the patient. Most attention has focused on genetic polymorphisms for cytokines, and other genes involved in immune responses. It is now well recognized that there is wide inter-individual variation in the level of production of various pro- and anti-inflammatory cytokines.44 Part of the explanation for these inter-individual differences lies in the presence of functional genetic polymorphisms, most commonly in the regulatory region of various cytokine genes. Using sequence specific primer-polymerase chain reaction methodology, we have recently observed that “high producers” of the pro-inflammatory cytokine TNF-α, and “low producers” of the anti-inflammatory cytokine IL10, are more likely to experience recurrent cardiac rejection in the first year after transplantation.45 We have also investigated polymorphisms of other genes that may influence outcomes. Of particular interest are genes that influence individual responses to various immunosuppressive drugs. The multi-drug resistance gene MDR1, encodes P-glycoprotein, a transport membrane glycoprotein that acts to expel many immunosuppressive drugs to the outside of the cell. We have recently noted that certain polymorphisms of this gene, at exons 21 and 26, correlate with increased chance of weaning children from corticosteroids within the first year after transplantation.46 It is important to note that most African-American individuals carry the polymorphisms at these locuses that are associated with decreased immunosuppressive efficacy. It has long been known that the African-American population has poorer outcomes after transplantation, with worse profiles for rejection. These kinds of genetic studies hold promise that we will be able to predict the risk of an individual patient for acute rejection, and other outcomes, thus enabling us to tailor immunosuppressive therapy to the needs of the individual child.
The optimal method for diagnosing acute rejection remains one of the most controversial issues in the management of children undergoing transplantation. In most centers, as with adults, endomyocardial biopsy remains the gold standard for the diagnosis of acute rejection. A large number of studies in adults have investigated the role of echocardiography in the diagnosis of acute allograft rejection.47, 48 The almost universal use of protocols based on endomyocardial biopsy for surveillance in adult practice is testament to the fact that no echocardiographic parameter, or combination of parameters, appears able reliably and reproducibly to diagnose allograft rejection. Because of the inconvenience, greater technical challenges, and possible increased morbidity of biopsy in smaller children, there has been much interest in evaluating the role of echocardiography in children undergoing transplantation.49–52 A number of echocardiographic parameters have been investigated, including various measures of systolic and diastolic function, changes in left ventricular wall thickness and mass, and development of new mitral regurgitation or pericardial effusion. Standard M-Mode, cross-sectional, and Doppler studies,49–51 automated techniques to detect borders, and tissue characterization have all been evaluated.52, 53 Unfortunately, most studies have involved too few patients, or have not used endomyocardial biopsy as a concurrent gold standard. Boucek and colleagues did compare an echocardiographic scoring system to the results of endomyocardial biopsy in a group of children.49 Most of the biopsies were performed beyond the first few months after transplantation. It is difficult, therefore, to draw conclusions about the use of echocardiography for surveillance of rejection in the immediate period after transplantation, when patients are most at risk for the development of acute rejection. During this period, many other factors come into play that may effect cardiac function, dimensions, wall thicknesses, and mass. These include the known effects of cardiac hypoxia or ischemia and reperfusion on diastolic dysfunction, the effects of steroids on left ventricular wall thickness and mass, as well as the influence of mismatch between the size of the recipient and the transplanted heart on ventricular remodeling.54, 55 It is noteworthy that Santos-Ocampo and colleagues from St. Louis56 found M-mode echocardiography to be an unreliable means of diagnosing rejection in the first month after transplantation in infants. The controversy over the role of echocardiography for the diagnosis of acute rejection is far from resolved.
The child who has been transfused at the time of previous surgery, or to treat severe anemia, may become sensitized to a wide variety of human leukocyte antigens. These patients are at risk for the development of severe humoral rejection after transplantation.38 The management of the ambulatory candidate with an elevated panel reactive antibody screen remains controversial. Prospective cross matching of the candidate serum against donor lymphocytes is rarely feasible, since most donor hearts for children are procured from distant sites where recipient serum is not available. Attempts can be made to reduce the level of recipient anti-human leukocyte antigen antibodies. Strategies that have been used to achieve this include the use of intravenous pooled immunoglobulin,57 plasmapharesis,58 as well as agents that may inhibit production of antibodies by B cells.59, 60 Cyclophosphamide, azathioprine and mycophenolate mofetil have all been used in this regard.
In the critically ill child, a negative prospective cross match is unlikely to be achieved prior to the death of the patient. A decision must then be made as to whether the first compatible donor of suitable size and blood group will be used, irrespective of the potential result of cross-matching. These candidates can be managed with pretransplantation plasmapharesis, which can then be continued intraoperatively and postoperatively. This should minimize the risk of hyperacute rejection with immediate vascular thrombosis and graft failure. Ongoing problems related to humoral sensitization may occur, including severe acute rejection often associated with graft dysfunction. The endomyocardial biopsy will frequently show marked edema and endothelial activation, but with little cellular infiltrate. There are few experimental studies to guide the treatment of humoral rejection beyond plasmapharesis.61 Induction cytolytic therapy with anti-T cell antibodies may be used, since humoral responses may up-regulate cellular immune responses. Triple drug therapy is indicated, and cyclophosphamide, mycophenolate mofetil, or rapamycin are logical choices for adjunctive therapy in the immediate period after transplantation, since they may suppress production of antibodies with greater efficiency than azathioprine. Use of intravenous gamma globulin is considered once plasmapharesis is discontinued. Only one study has been reported in children using these types of strategies. Good intermediate term results were observed.62 It should be noted, however, that there is increasing evidence that antibodies play a role in the development of chronic rejection.63 Thus, although an aggressive approach to the management of the sensitized patient may allow for short and medium term survival, the long terms consequences of transplanting sensitized children, especially with a positive cross match, remain unknown.
Coronary arterial disease subsequent to transplantation is an accelerated vasculopathy that is the leading cause of death among late survivors of heart transplantation.2 The pathology differs somewhat from that of ischemic heart disease in the normal adult population.64 Typical allograft coronary arterial disease consists of myointimal proliferation that is concentric and involves the entire length of the vessel, including intramyocardidal branches. Eventually luminal occlusion occurs (Fig. 3a,b). There may also be a pronounced inflammatory component that is not typically seen in atherosclerotic disease. Progression of disease also tends to be more rapid subsequent to transplantation.

Figure 3. Chronic cardiac rejection. (a) Selective angiogram of left coronary artery obtained 3 years after transplantation in a 10 year-old child with diastolic dysfunction. Although post-transplantation coronary arterial disease is generally a diffuse process, severe focal lesions (arrows) may also be seen. (b) Histological section of an epicardial coronary artery with advanced vasculopathy. There is severe luminal narrowing secondary to intimal proliferation (*).
In adults, from one-third to half of recipients have angiographic or autopsy evidence of this condition by 5 years after transplantation. “Moderate-to-severe” disease occurred in one-sixth of patients registered in the multi-centric adult Cardiac Transplant Research database maintained at the University of Alabama, Birmingham. Using the same method of diagnosis, the incidence at five years after transplantation was 6% among recipients in the Pediatric Heart Transplant Study.65 Overall, the incidence of any angiographic evidence of coronary disease was 3, 12, and 20% at 1, 3 and 5 years after transplantation of children in this dataset. Freedom from chronic rejection at Children's Hospital of Pittsburgh is approximately 90% at 5 years after transplantation (Fig. 4). Clearly, the incidence of this serious complication will be dependent on the method of survey. Most studies have relied on angiography for diagnosis. This will tend to underestimate mild disease that may be easily detected by intravascular ultrasound.

Figure 4. Probability of freedom from chronic rejection due to coronary arterial disease among children undergoing primary cardiac transplantation at Children's Hospital of Pittsburgh from 1982 to 2002.
Both immune and non-immune mechanisms likely contribute to the development of coronary arterial disease, though immune mechanisms are probably of central importance in children. Even neonates, with no traditional risk factors, are now recognized to be at risk for the development of coronary arterial disease. The immunobiology of chronic rejection is discussed in detail elsewhere.66 Identification of specific risk factors for development of disease in children has recently been investigated. Among 1032 recipients at 18 centers in the Pediatric Heart Transplant Study, older recipient age, older donor age, especially beyond 30 years, and greater number of episodes of rejection in the first year, were the main risk factors for development of coronary arterial disease.65 Donor age beyond 30 years tripled the risk of disease at 5 years after transplantation.
The recipient will often not experience ischemic chest pain, since the heart is denervated. Heart failure, syncope or sudden death is often the initial presentation.67 Standard treadmill and nuclear scans for the detection of coronary arterial disease are generally insensitive in this population. It has been suggested that dobutamine stress echocardiography is the most promising noninvasive technique. Only limited experience has been reported at this time in children.68, 69Furthermore, many children do not agree that this is a non-invasive test! Nausea and palpitations are common, and intravenous access is required. The sensitivity, specificity, and predictive values for detection of disease in adults are fairly high, and superior to other non-invasive methods. In addition, serial dobutamine stress studies have proved useful in predicting acute cardiac events in adults.70 Undoubtedly further experience in this field with children will be forthcoming in the future.
Angiography has traditionally been the gold standard for detection of coronary arterial disease in children and adults. It does, however, carry serious limitations, beyond the obvious facts that it is invasive and expensive as a screening tool. Angiography is insensitive, though severe epicardial disease that portends a poor prognosis is readily apparent. It has proven very difficult to predict prognosis for patients with mild to moderate angiographic disease, many of whom will do well for several years after diagnosis. Some pediatric centers advocate listing for retransplantation at the time of diagnosis of any angiographic abnormality because of the progressive nature of the disease, and the tendency for angiography to underestimate the degree of vessel involvement. We are more conservative, and would re-list only children with either abnormal hemodynamics, rapid progression, a positive stress echocardiogram, or decreased left ventricular systolic function. Since little data is currently available regarding natural history in children, it seems appropriate to follow patients closely with serial angiography and stress echocardiography when “mild” disease is identified.
Intravascular coronary ultrasound has the greatest sensitivity for detection of graft vasculopathy. Studies in adults have reported the frequent occurrence of abnormalities on intravascular ultrasound, despite angiographically normal appearances of the coronary arteries.71 Two studies from large programs involving children have also shown a discrepancy between findings from coronary angiography and intravascular ultrasound, abnormalities commonly being predicted ultrasonically when angiography is “normal”.72, 73 Interestingly, in the Loma Linda experience, maximal intimal thickness was greater in children transplanted in infancy and early childhood compared to those transplanted as neonates.72 This observation correlates with the recently reported survival advantage for neonatal recipients.74 At the present time, however, intravascular ultrasound should be considered experimental in children, with the risks of the procedure not yet being adequately established in this population.
No effective treatment exists for established coronary arterial disease. Bypass grafting, and coronary angioplasty, have limited utility due to the diffuse nature of the disease. Similarly, stenting is of limited utility due to intimal proliferation through the stent, and due to progression of the disease in other areas. Rapamycin coated stents have shown great promise for preventing restenosis in ischemic heart disease.75 We are currently using them in selected children after transplantation with focal lesions. Re-transplantation is limited due to the shortage of donors, especially for the older child who will be competing with adults for donor organs.
Several pharmacological approaches have been used for prevention of coronary arterial disease after transplantation. Anecdotal reports, small clinical trials in adults, and experimental models in animals suggest that a large number of agents may be potentially useful in prevention of graft vasculopathy and coronary arterial events. These include calcium antagonists, angiotensin converting enzyme inhibitors, anti-oxidants such as vitamin E, “statins”, aspirin, and antiproliferative agents such as mycophenolate mofetil and rapamycin. The latter agent is of particular interest, since it has been shown to prevent the development and progression of graft vasculopathy in a number of animal transplantation models, including primates.76 Unfortunately, it will take many years before it is known whether this agent, or any other, can reduce the incidence and severity of chronic rejection in human recipients of transplanted hearts. Furthermore, a major side effect of this agent is hyperlipidemia, widely considered to be a risk factor for graft coronary arterial disease. Since adherence to complex medical regimens is a significant challenge for many patients and families, it is hard to justify routine use of all these agents in the absence of proof of efficacy. On the other hand, it must be recognized that large-scale multicentric trials of most of these therapies are unlikely to ever be performed, especially in children.
The spectrum of infections after transplantation in children, and their prevention and treatment, has been the focus of a recent review.77 An increased prevalence of all forms of infection is seen. Most infections are caused by pathogens that also cause infection in non-immunocompromised children. Common examples include respiratory viruses, Streptococcus pneumoniae, and Varicella Zoster virus. All infections that may be seen in non-immunocompromised patients can cause greater disease severity in the recipient of a transplanted heart, and, thus, strategies to achieve prevention and early treatment are imperative. Of particular note in this respect are infections due to cytomegalovirus and Epstein-Barr virus, which only rarely cause severe disease in the immunocompetent host. More rarely, opportunistic infections are seen such as that due to Pneumocystis carinii (Fig. 5). Although most infections are well tolerated, it should be noted that infection ranks comparable to early graft failure and rejection as the main causes of death after heart transplantation in children (Table 1).

Figure 5. Rapid evolution of infection with Pneumocystis carinii in a 15 year-old child presenting with cough. The chest radiographs are taken 48 hours apart.
Table 1. Causes of death after pediatric heart transplantation. Data from 1114 primary transplants in the Pediatric Heart Transplant Study 1993–2000 (total 248 deaths). (Used with permission.)

Relatively few major advances in the prevention and management of infection after transplantation have been realized in recent years. Two important exceptions are infections with cytomegalovirus and Epstein-Barr virus (see below). Cytomegalovirus is an important cause of morbidity after solid organ transplantation. Symptomatic disease in children is relatively unusual.78 Some groups have suggested that cytomegalovirus infection is a risk factor for development of coronary arterial disease.79 It is unknown if this is true in children, though it is of interest to note that, in a recent intravascular ultrasound study from Loma Linda, an increase in maximal intimal thickness and intimal index correlated with cytomegalovirus seropositivity.72 Advances in diagnosis, prevention, and treatment of cytomegalovirus have been realized in the last decade. These include widespread availability of ganciclovir as an intravenous and oral antiviral agent, the development of cytomegalovirus immune globulin preparations, and assays for rapid diagnosis of active infection from peripheral blood samples, such as pp65 antigenemia test and polymerase chain reaction detection of viral genome. The optimal strategies for preventing infection with cytomegalovirus remain to be determined in children.
The last few years have seen major advances in our understanding of the nature of Epstein-Barr infection in the immunocompromised host. The immunobiology, nomenclature, management, and possibilities for prevention of symptomatic Epstein-Barr virus disease and lymphoproliferative disorders occurring after transplantation have been the topic of several recent reviews.80–85 Recent review of the Pediatric Heart Transplant Study database identified 56 cases among 1184 primary transplants (4.7%) at 19 North American centers.86 Almost nine-tenths were driven by Epstein-Barr virus. The pathology of early onset disease was usually polymorphic, though frequently monoclonal, and almost invariably associated with primary infection with Epstein-Barr virus. Post-transplant lymphoproliferative disorders developing late, usually beyond 3 years, may be polymorphic, but are often monomorphic and lymphomatous.
The clinical manifestations of lymphoproliferative disorders occurring after transplantation are protean.80 Fever and malaise are the most common presenting symptoms in children. In contrast to other transplanted solid organs, disease is rarely seen within the allograft. The majority of children who develop these disorders are diagnosed with lung nodules, mediastinal or abdominal adenopathy, or gastro-intestinal involvement. Abdominal pain, vomiting, and diarrhea are the major signs of gastro-intestinal involvement. Bleeding and perforation may also occur.
The recent development of quantitative Epstein-Barr virus–polymerase chain reaction on peripheral blood samples has proven to be a very useful simple technique for diagnosing and monitoring disorders driven by this virus.81, 82 The development of very high viral loads should alert the physician to look for sign or symptoms of disease, even in the asymptomatic patient. We routinely monitor seronegative patients at transplantation who are at risk by monthly polymerase chain reaction for the first 6–12 months after transplantation, and at the time of development of symptoms suggestive of the disease. This has enabled us to diagnose primary Epstein-Barr virus infection at a very early stage, that is before the development of symptoms. Studies are underway to determine if preemptive decrease in immunosuppression, and/or use of immunoglobulin preparations with high titers of anti-Epstein-Barr virus antibodies, can safely reduce the risk of lymphoproliferative disease when high viral loads are detected in an otherwise healthy child. We have also used weekly monitoring of polymerase chain reaction to assess response to treatment in patients with symptomatic infection by the Epstein-Barr virus or those with lymphoproliferative disease.80–82 Response to therapy has been associated with a precipitous fall in viral load, even before radiographic improvement has occurred. Furthermore, preliminary experience has suggested that monitoring of the viral load may help predict time to development of rebound rejection after cessation or reduction of immunosuppression. When very high viral loads persist, we have not observed acute rejection in recipients of either transplanted hearts or livers having lymphoproliferative disorders. Fall of the viral load to <200 genome copies/105 peripheral blood mononuclear cells has been associated with development of rebound rejection in several patients. This preliminary experience suggests that serial monitoring by quantitative polymerase chain reaction may help determine appropriate changes of immunosuppressive therapy and timing of endomyocardial biopsy in patients being treated for lymphoproliferative disorders occurring subsequent to transplantation.
The mainstay of treatment in children remains reduction or temporary discontinuation of immunosuppression. In our recent experience, this course of action is associated with “cure” in approximately four-fifths of cases with polymorphic histology. Rebound rejection is common, and must be sought by increased echocardiographic and endomyocardial biopsy surveillance. It has been rare for the disorders to recur, even if treatment with pulsed corticosteroid therapy for rebound rejection is required. The monomorphic forms are less likely to respond to a reduction in immunosuppression, but it has been our practice to attempt this as first line therapy in selected patients, since complete remissions may be observed with this therapy alone. Monomorphic disease may require treatment with conventional chemotherapeutic agents, as do overtly malignant lymphomas, such as Burkitt's lymphoma.
Several other therapies have been proposed in recent years. Two therapies are of particular interest, as they may effectively treat the condition without requiring global immunocompetence to be reestablished. In this way, rebound rejection may be avoided. The first is use of monoclonal antibodies directed against B cell antigens. More than nine-tenths of lymphoproliferative disorders are of recipient B cell origin after heart transplantation. In the United States of America, one such agent, rituximab (Rituxan, Genentech Inc./IDEC pharmaceuticals) is approved for treatment of certain types of adult B cell non-Hodgkin lymphomas. This is a human/mouse chimeric monoclonal antibody directed against the CD20 antigen carried on almost all B cells. We have found that most lesions express this antigen. Several reports have now suggested that this agent may be a useful therapy for B cell post-transplant lymphoproliferative disorders (Fig. 6).87–89 A trial of this agent in children with refractory disease is in progress. A second novel approach is cellular immunotherapy, whereby the patient is given an infusion of cytotoxic T-lymphocytes directed against Epstein-Barr virus-specific antigens. This should result in control of the proliferation of the B cells infected with the Epstein-Barr virus, but without the risk of rejection. The use of Epstein-Barr virus-specific cytotoxic T cell therapy has already been developed, and applied successfully, for the management of post-transplant lymphoproliferative disorders in recipients of transplanted bone marrow.90 Efforts to achieve this in recipients of transplanted solid organs are under way in several centers, and preliminary data suggests this should be feasible.91

Figure 6. Response of refractory post-transplant lymphoproliferative disorder to the anti-CD20 monoclonal antibody rituximab. The disease progressed despite withdrawal of immunosuppression (panels a and b) (arrows), but responded completely to a 4-week course of the antibody (panel c). Panel (d) shows a histological section of the lesion stained with an anti-CD20 antibody. There is dense staining of the membranes of the B lymphocytes for CD20, the target of the therapeutic antibody.
Reports of malignant neoplasia other than lymphoma are rare in transplanted children.92 Unfortunately, the risk of malignancy in immunosuppressed patients appears to persist through the lifetime of the patient. This is of particular concern for children destined to receive lifelong immunosuppression. Other than lymphoma, squamous cell carcinomas and other skin neoplasia are the commonest malignancies seen after heart transplantation.
Our focus on rejection, infection, and malignancy emphasizes the careful balance that must be achieved between over and under-immunosuppression. In addition to these complications, recipients experience a wide array of other complications that are unrelated to their immune status. Most reflect end-organ toxicities of immunosuppressive therapies.93 Systemic hypertension is a common complication, especially in those maintained on cyclosporine-steroid based immunosupression. It generally responds to standard anti-hypertensive agents, but on occasion can be quite resistant to treatment. Hyperlipidemia is also common in children after heart transplantation.94, 95 Recent data show that children treated with cyclosporine experience more adverse lipid profiles compared to those treated with tacrolimus, and this effect occurs independent of the effects of corticosteroids.96 Treatment with statins is generally quite effective, though rare cases of rhabdomyolysis indicate the need for careful monitoring during therapy. Dose-related and idiosyncratic neurological complications of current immunosuppressive regimens include seizures, tremors, paraesthesias, and even encephalopathies.97 Common metabolic complications include hyperkalemia, hyperuricemia, hypomagnesemia and, most importantly, diabetes mellitus.98 The relative diabetogenic effect of cyclosporine and tacrolimus is the subject of considerable controversy.99 We have found that the development of diabetes is rare subsequent to transplantation in pre-adolescents.100 Adolescent age, obesity, and the need for high doses of corticosteroids, have been major risk factors in our population. The diabetes reflects both high peripheral insulin resistance and pancreatic insufficiency. This complication is likely to become less frequent with newer immunosuppressive regimens that allow for lower doses of calcineurin inhibitors and corticosteroids.
One of the most worrisome end-organ toxicities is that of progressive renal dysfunction due to calcineurin inhibitor renal toxicity. In our own experience, up to one-twentieth of recipients have developed severe renal dysfunction or end-stage renal failure. We have observed no significant difference in renal function between cyclosporine and tacrolimus-based immunosuppressive regimens up to 7 years after transplantation.101 With increasing numbers of patients surviving into the second decade after transplantation, the number of patients developing end-stage renal failure will inevitably increase. We have also found that estimates of creatinine clearance based on serum creatinine levels significantly underestimate the extent of renal dysfunction when compared to nuclear medicine measurements of glomerular filtration rate.101 Indeed, approximately two-fifths of our patients during long-term follow-up have glomerular filtration rates >2 standard deviations below mean normal (Fig. 7). Increased use of adjunctive therapies without renal toxicity, for example rapamycin and mycophenolate mofetil, should allow for reduced dosing of tacrolimus and cyclosporine. This will hopefully reduce the extent of chronic nephropathy associated with these agents. Some groups have even attempted calcineurin free immunosuppressive regimens, though this has not yet been attempted in cardiac recipients. Use of multiple immunosuppressive agents with differing end-organ toxicities, each at low dosage, represents a logical approach to minimizing side effects while maintaining immunosuppressive efficacy. Such complex regimens are only suitable for the most motivated and compliant of patients. We struggle with compliance using the simplest of monotherapy regimens in our population of adolescents. Studies are also underway on a new generation of potent calcineurin inhibitors that appear to exhibit minimal renal toxicity.102 This represents a potential major advance in immunosuppressive therapy.

Figure 7. Renal function during long-term follow-up of children undergoing heart transplantation at Children's Hospital of Pittsburgh. Glomerular filtration rate (GFR), assessed using the Tc-99m DTPA technique, is shown for 69 patients with serum creatinine below 2 mg/dl. Although end-stage renal failure is rare, approximately two-fifths of recipients have filtration rates more than 2 standard deviations below the mean normal.
There has been a major increase in the number of new immunosuppressive agents available in the last decade (Table 2). This reflects the increasing interest in transplantation by pharmaceutical companies now that solid organ transplantation is an accepted therapy for end-stage organ failure, and long-term survival is feasible. Our increasing understanding of the molecular and cellular basis for acceptance and rejection of the transplanted organs has allowed for the rational development of new drugs. We can anticipate that many more agents, particularly monoclonal anti-bodies, will be developed in the coming years based on a detailed understanding of the target molecules and receptors. A comprehensive review of all aspects of immunosuppressive therapy is outside the scope of this review. Instead, I will briefly review how immunosuppression has been used in children, with emphasis on the role of new agents.
Table 2. New Food and Drug Administration approved immunosuppressive agents introduced into the United States in the last decade.

The use of monoclonal or polyclonal induction therapy, such as OKT3 or antithymocyte globulin preparations, remain highly controversial in both adults and children. Single and multicentric randomized trials in adults have failed to resolve the question, and no randomized multicentric studies have been performed in children. In adults, the main benefit has been to delay onset of rejection, but no enduring benefits have been established. The influence of induction therapy was investigated in a recent retrospective multicenter study of 465 children.103 Patients receiving polyclonal rabbit antithymocyte serum had overall lower mortality and reduction in deaths due to rejection when compared to patients receiving no induction or OKT3. This study was not randomized, and it should be noted that the polyclonal antibody was used in some of the most experienced centers for transplantation of infants and children in the United States of America. This may have contributed to the improved survival. It is therefore hard to draw firm conclusions about the efficacy of antibody induction. The results do, however, stress the need for randomized studies of induction therapy in children, both for establishing efficacy and safety. There are no reported trials of induction therapy with the two new monoclonal antibodies directed against the α-chain of the interleukin-2 receptor (basiliximab and daclizumab) in children undergoing heart transplantation. In adult transplantation, these agents have proven to have excellent profiles for adverse events, and have been shown to reduce the frequency and severity of episodes of early acute rejection.104, 105 They are likely to be increasingly used in children in the next few years, and may help facilitate avoidance of steroids, or their early withdrawal.
All immunosuppressive regimes in children are based around the use of a calcineurin inhibitor, either cyclosporine or tacrolimus. Approximately four-fifths of recipients currently receive cyclosporine, with the remainder being on tacrolimus.2 The proportion of patients receiving tacrolimus is gradually increasing, though it should be noted that the two agents have not been compared in randomized trials in children after transplantation of thoracic organs. Most centers also use a second or third agent.2 Azathioprine and corticosteroids are the most commonly used adjunctive therapies, though there is increasing use of mycophenolate mofetil. There have been no randomized trials of mycophenoate in children, but there has been one large multicentric trial in adults after cardiac transplantation.106
This was a randomized, blinded study from 28 centers in Australia, Europe, and North America comparing therapy consisting of cyclosporine, corticosteroids, and azathioprine to therapy with cyclosporine, corticosteroids, and mycophenolte mofetil. There was a very high withdrawal rate from the study, approximately two-fifths of each group. Survival and rejection were similar in the 2 randomized groups. In patients who actually received mycophenolate, there was a statistically significant reduction in mortality at 1 year, 6.2% as opposed to 11.4% for those receiving azathioprine. Survival at 3 years was 88% in those treated with mycophenolate, and 82% in the patients getting azathioprine. There was also a reduction in the requirement for treatment of rejection at 1 year, and a trend to fewer patients having greater than, or equal to, grade 3A rejection, or needing cytolytic agents in the group treated with mycophenolate. Increasing use of this agent can be anticipated for children in the coming years.
A number of centers have tried to minimize the use of corticosteroids, in an attempt to reduce corticosteroid morbidity.107–109 Approximately three-quarters of children receive corticosteroids at the time of hospital discharge, but this has fallen to half by 3 years from transplantation.2 There are no randomized trials of routine use of steroids versus their avoidance in children subsequent to heart transplantation. It is clear, however, that withdrawal is feasible in many, if not most, children in the medium term of 6 months to 2 years after transplantation.15, 110 Use of antibody induction therapy, tacrolimus as primary immunosuppressant, and newer adjunctive agents such as mycophenolate mofetil and sirolimus, may enhance the ability to achieve complete avoidance or early withdrawal of steroids.
Rapamycin, or sirolimus, is a newly approved agent with potent immunosuppressive properties.111 The main concerns have been hyperlipidemia and marrow suppression. Need for discontinuation of this agent due to side effects has been rare in adults, but a significant proportion of patients will require statins to control hyperlipidemia. There is increasing evidence that this agent can be safely used with both cyclosporine and tacrolimus, despite the fact that it targets the same binding protein as tacrolimus. A plethora of studies in adults is under way to assess the role of rapamycin agents as adjunctive therapies with calcineurin inhibitors after all forms of solid organ transplantation. Some groups have studied use of high dose sirolimus with complete calcineurin avoidance after renal transplantation. Such trials are unlikely in cardiac transplantation, since the consequences of failure of this therapy could be catastrophic. There is likely, nonetheless, to be increasing interest in this agent as a calcineurin-sparing therapy for patients with serious complications of tacrolimus or cyclosporine therapy, such as significant renal dysfunction.
The benefits of tacrolimus, as opposed to regimes based on cyclosporine, remains one of the most controversial issues in transplantation in children. Most experience has been gained at the University of Pittsburgh, where tacrolimus has been used since 1989.15, 98, 100, 101, 112–116 This extensive experience in over 200 children suggests a slight delay in time to first rejection, less episodes of rejection in the first year, and less need for lympholytics beyond the early period after transplantation. Furthermore, there was lower requirement for long-term steroid therapy, and many patients were managed with tacrolimus monotherapy by the end of the first year.15 The cosmetic results were also much better, with no evidence of hirsuitism, coarsening of facial features, or gingival hyperplasia. Renal function has been comparable between children treated with tacrolimus and cyclosporine,101 while hypertension and hyperlipidemia tend to be less prevalent in those treated with tacrolimus. One very important caveat should be noted. The comparison with patients receiving cyclosporine was based on historical controls, and other factors may have contributed to improved profiles for rejection in the more recent cohort treated with tacrolimus. Unfortunately, there is little experience to draw on from adults. No pivotal trials comparing these two agents have been performed in adults undergoing cardiac transplantation.
Data from the International Society for Heart and Lung Transplantation show 1 and 5 year survival of 78% and 67%, respectively.2 Data from the Pediatric Heart Transplant Study show 1 and 5 year survival for children undergoing transplantation between 1993 and 2000 of 84% and 73% respectively among 1114 recipients of primary grafts at 20 North American Centers (unpublished data). In our own experience, we have observed a progressive improvement in survival of both the transplanted heart and the patient over the past 20 years (Fig. 8), especially for those having congenital heart disease.15, 16 Three-year survival currently exceeds 90%. Causes of death, and risk factors for survival, have been analyzed within the Pediatric Heart Transplant Study.117 The major causes of death are early graft failure, infection, and acute rejection (Table 1). Together, these account for almost half of all deaths after transplantation. Interestingly, age at transplantation was not a significant risk factor for survival in the analysis of the data from 1993 through 2000 analysed from the consortium of centers in the United States of America, although a slight trend to lower survival was seen for infants. This difference was no longer apparent by 3 years from transplantation. Beyond the first 6 months of life, survival was almost identical among patients hospitalized versus those cared for at home at the time of transplantation. A further point of interest was the observation that survival after transplantation was not influenced by prolonged ischemic time >300 minutes. This observation has led some programs to procure organs from distances in excess of 2000 miles. Diagnosis of hypoplastic left heart syndrome, or other congenital heart disease, was significantly associated with worse outcome in the first few years after transplantation (Fig. 9). As noted previously, comparable outcomes among those with and without congenital heart disease have been observed in several experienced centers.16, 17

Figure 8. Evolution of survival after cardiac transplantation during childhood at Children's Hospital of Pittsburgh, over the period 1982–2002.

Figure 9. Survival stratified by the cardiac diagnosis prior to transplantation. Data used with permission from the Pediatric Heart Transplant Study over the period 1993–2000.
Most recipients are well rehabilitated, and are in Class I of the classification of the New York Heart Association. Full time attendance at school is achieved in almost all patients within a few months of transplantation. We have noted that maximum capacity for physical work, and peak heart rate, are only approximately two-thirds of predicted, and peak oxygen consumption just exceeds half of values in normal control.117, 118 The reasons for these changes are complex, and include chronotropic incompetence and diastolic dysfunction of the allograft.
Many recipients experience difficulties in psychosocial adjustment, with adolescents being most vulnerable.119, 120 Few studies have addressed the magnitude of this problem, and even fewer have sought solutions. The topic of non-adherance with medical therapy is another seriously neglected area of research, though a few centers have started formally to evaluate this problem.120, 121 Many centers have reported that incomplete adherence with immunosuppressive therapy is the leading cause of death late after transplantation. Our own experience supports this contention. The idea that these are unglamorous or unworthy areas for clinical research, and not worthy of funding, must be challenged if progress is to be made. Further research is also required in the areas of somatic growth, pubertal development, and cognitive function after transplantation of the heart in children.122
Donor specific tolerance after transplantation can be defined as a state in which the recipient will permanently accept the allograft without the need for long-term anti-rejection therapy, yet retain normal host immune responses to antigens other than those of the donor. Thus, the risks of infection, lymphoproliferative disorders, and malignancy are avoided. Tremendous effort has been expended in recent years to understand how tolerance can be achieved.123–125 Many experiments in small animals, particularly rodents, have now been extended to subhuman primates. It is, of course, necessary that this tolerance be robust and long lasting. Pertubations of the immune system of the recipient, such as immunological responses to everyday infections, cannot result in loss of tolerance, or else rapid rejection of the transplanted organ will ensue. It has become apparent that, as one advances up the evolutionary tree, it becomes more difficult to achieve stable tolerance. It is likely that we are several years away from this goal in humans. The complex molecular and cellular events involved in induction of tolerance, nonetheless, are becoming clearer, and this will greatly enhance our ability to achieve this goal. In simple terms, three important mechanisms appear to be involved in establishment of T cell tolerance. These are clonal deletion, involving thymic or peripheral deletion of donor specific T cells, anergy involving functional inactivation of T cell clones, and suppression or regulation of donor-specific T lymphocytes by other, poorly characterized, immunoregulatory cells or humoral factors.
In practice, these multiple cellular mechanisms are not mutually exclusive. There is good evidence from many animal studies that several of these mechanisms may be involved in any given model. In particular, different mechanisms may be involved in the induction and maintenance phases of the tolerant state. Our state of knowledge of this field, and the potential huge benefits to patients from induction of tolerance, have lead a number of groups to suggest that the time is right to commence human trials in this field. Initial emphasis has been placed on studies in transplantation of kidneys and pancreas in adults, in part because these are not life-supporting organs, and return to dialysis is possible if the strategies fail. Children also offer some special opportunities. Firstly, induction may be most readily achieved in the neonate, and transplantation in the neonatal and early infant period is extremely rare for organs other than the heart. In addition, the median sternotomy required for heart transplantation exposes the thymus gland, allowing for potential manipulation of this organ for induction of tolerance. In many small animal models, intra-thymic inoculation of donor antigens, particularly bone marrow, results in development of donor specific tolerance.126 A trial of thymic tolerance by inoculation of the thymus gland with donor bone marrow concomitant with heart transplantation in children is currently underway at Children's Hospital of Pittsburgh.
Other strategies for inducing transplantation tolerance are summarized elsewhere.123–125 Blockade of co-stimulatory pathways, the “second signal” for T cell activation, with monoclonal antibodies looks promising.127 This form of peripheral tolerance may be inhibited by concomitant usage of calcineurin inhibitors. This poses serious problems for design of clinical trials, since withholding calcineurin inhibitors may have catastrophic consequences if the anticipated effects of costimulatory blockade are not realized. Another promising model is that of mixed hematopoietic chimerism.123 In this model, persistence of donor and recipient hematopoietic cells in the recipient allows for long-term allograft acceptance. Early clinical trials involving peripheral infusion of unmodified donor bone marrow at the time of transplantation in humans has not resulted in tolerance to heart and lung allografts, though some donor specific immunomodulation may occur.128, 129 Long-term engraftment of donor cells at sufficient level to allow for graft acceptance without long-term immunosuppression requires preconditioning of the recipient to “make space” for the donor hematopoietic tissue to engraft. Engraftment can be facilitated by use of non-lethal irradiation, chemotherapeutic agents, and or use of high dose or repeated doses of donor bone marrow. All these strategies will come at a price, in particular an increased risk of severe infection and or graft-versus-host disease. With excellent short and medium term results being achieved for many solid organ transplants, including transplantation of the heart in children, the ethical issues in designing trials for induction of tolerance in humans are self-evident.
In the last two decades, we have witnessed great improvements in outcomes after transplantation of the heart in children. Surgeons have risen to the challenge of transplanting the heart in recipients having the most complex anatomy, and have achieved great success in reducing early mortality. Mechanical support as a bridge to transplantation remains in its infancy, with few devices available for use in children. Work in this area is hampered by economic considerations. Major advances have also occurred in recent years in our understanding of the immune response to solid organ allografts. This is resulting in improved patient care. New immunosuppressive agents can now be designed to target molecules and receptors that are known to be of central importance in the immune response to the allograft. There will certainly be an explosion in the number of new drugs available for clinical use in the next few years. Our understanding of risk factors for individual outcomes is also improving, including new knowledge about genetic factors. Improved understanding of the immune response to infection with the Epstein-Barr virus, combined with new strategies for monitoring and treatment, suggest that lymphoproliferative disorders may be prevented after transplantation, or more satisfactorily treated in the years to come. It is becoming increasingly apparent that successful induction of tolerance may become a reality in the clinic in the next decade, potentially transforming transplantation from a palliative to a “curative” therapy, with expectations for indefinite survival of the transplanted organs.

Indications for heart transplantation among 169 recipients, undergoing 180 transplantations, at Children's Hospital of Pittsburgh over the period 1982–2002.

Probability of freedom from acute rejection among 1114 primary transplantations recorded in the files of the Pediatric Heart Transplant Study, from 1993 to 2000. The material is used with permission of the Pediatric Heart Transplant Study.
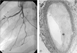
Chronic cardiac rejection. (a) Selective angiogram of left coronary artery obtained 3 years after transplantation in a 10 year-old child with diastolic dysfunction. Although post-transplantation coronary arterial disease is generally a diffuse process, severe focal lesions (arrows) may also be seen. (b) Histological section of an epicardial coronary artery with advanced vasculopathy. There is severe luminal narrowing secondary to intimal proliferation (*).

Probability of freedom from chronic rejection due to coronary arterial disease among children undergoing primary cardiac transplantation at Children's Hospital of Pittsburgh from 1982 to 2002.
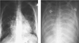
Rapid evolution of infection with Pneumocystis carinii in a 15 year-old child presenting with cough. The chest radiographs are taken 48 hours apart.

Table 1.

Response of refractory post-transplant lymphoproliferative disorder to the anti-CD20 monoclonal antibody rituximab. The disease progressed despite withdrawal of immunosuppression (panels a and b) (arrows), but responded completely to a 4-week course of the antibody (panel c). Panel (d) shows a histological section of the lesion stained with an anti-CD20 antibody. There is dense staining of the membranes of the B lymphocytes for CD20, the target of the therapeutic antibody.

Renal function during long-term follow-up of children undergoing heart transplantation at Children's Hospital of Pittsburgh. Glomerular filtration rate (GFR), assessed using the Tc-99m DTPA technique, is shown for 69 patients with serum creatinine below 2 mg/dl. Although end-stage renal failure is rare, approximately two-fifths of recipients have filtration rates more than 2 standard deviations below the mean normal.

Table 2.

Evolution of survival after cardiac transplantation during childhood at Children's Hospital of Pittsburgh, over the period 1982–2002.

Survival stratified by the cardiac diagnosis prior to transplantation. Data used with permission from the Pediatric Heart Transplant Study over the period 1993–2000.