Although the modification of the classical Blalock-Taussig shunt using a polytetrafluoroethylene conduit (W.L. Gore Inc., Elkton, MD, USA) remains the systemic-to-pulmonary arterial shunt of choice in our institution, we encountered a subset of patients which we considered unsuitable for this approach. Hence, we palliated them by modifying still further the so-called modified Blalock-Taussig shunt. This was achieved by interposing a short segment of polytetrafluoroethylene graft between the aortic arch or its branches and the intrapericardial portion of pulmonary arteries closest to the bifurcation of the pulmonary trunk (Fig. 1). Our present study was undertaken to evaluate the adequacy of palliation achieved with these shunts.
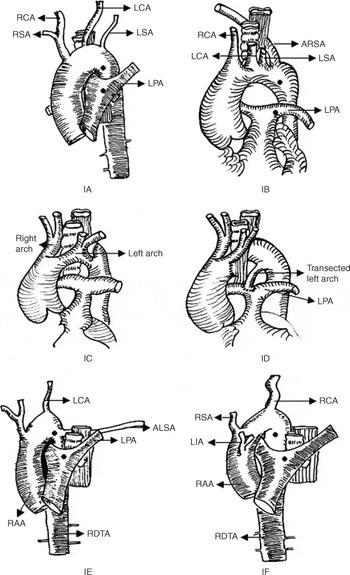
Figure 1. Diagramatic representation of various types of anomalies of the aortic arch and its branches necessitating our modification of the modified Blalock-Taussig shunt.
Figure 1. [bull ] – the proposed sites of aorto-pulmonary anastomosis; 1A – Left aortic arch, left descending thoracic aorta, hypoplastic right and left subclavian arteries; 1B – Left aortic arch, left descending thoracic aorta, anomalous retroesophageal right subclavian artery; 1C – Double aortic arch, dominant right arch, hypoplastic left arch; 1D – Systemic-pulmonary artery anastomosis utilizing the transected non-dominant portion; of the left arch; 1E – Right aortic arch, right descending thoracic aorta, anomalous retroesophageal left subclavian artery; 1F – Right aortic arch, right descending thoracic aorta, mirror-image branching of the aortic arch vessels.
Figure 1. Abbreviations: ALSA: Anomalous retroesophageal left subclavian artery, ARSA: Anomalous retroesophageal right subclavian artery, LIA: Left brachiocephalic artery, LCA: Left carotid artery, LPA: Left pulmonary artery, LSA: Left subclavian artery, RCA: Right carotid artery, RSA: Right subclavian artery, RAA: Right aortic arch, RDTA: Right descending thoracic aorta.
Patients and methods
For uniformity with other studies, we have used the following definitions:
Modified Blalock-Taussig shunt: The shunt was considered a modified Blalock-Taussig shunt if an expanded polyterafluoroethylene graft was inter-posed between the subclavian and pulmonary arteries. All patients having shunts placed between the brachiocephalic artery and the pulmonary arteries are included within this definition.
Conventional central aortopulmonary shunt: All modifications of aorto-pulmonary shunts, including those proposed by Waterston, Cooley, Potts, Gazanigga, Amato, and Mee, are grouped under the heading of conventional central aortopulmonary shunts.
The modification of the modified Blalock-Taussig shunt: The systemic-to-pulmonary arterial shunt was considered to have been further modified when the proximal end of the polyterafluoroethylene conduit was anastomosed to the aortic arch or its branches other than the subclavian or brachiocaphalic artery or proximal right descending thoracic aorta, and the distal end was anastomosed intrapericardially to the pulmonary trunk or pulmonary bifurcation as opposed to the right or left pulmonary artery.
The group studied included all patients in whom we modified still further the placement of the prosthetic graft as stated above. We also included 3 patients in whom the transected non-dominant end of a double aortic arch was utilized for the systemic-to-pulmonary arterial connection. Patients undergoing “conventional central aortopulmonary shunting procedures” are not included under this definition.
Disproportionately small right and left subclavian arteries: How small is too small? It is usually the length of the pulmonary artery to which the shunt is being anastomosed which limits the size of graft that can be implanted when constructing a modified Blalock-Taussig shunt. This is particularly true on the side of the aortic arch, where one can extend the anastomosis onto the origin of the subclavian artery from the arch, and distally for considerable distance.
Hypoplastic pulmonary artery: Pulmonary arterial diameters were estimated by echocardiography, angiography, and also at operation. The pulmonary arteries were considered as hypoplastic, and inadequate for complete biventricular or functionally univen-tricular repair, in the presence of one or more of the following:
- Ratio of diameter of the pulmonary arteries to the aorta of less than 0.75.
- McGoon's ratio of less than 1.01
- Nakata's pulmonary arterial index of less than 100 millimetres squared, less than 200 millimetres squared, or less than 250 millimetres squared, all normalized for body surface area, patients with tetralogy of Fallot, those future candidates for a Rastelli procedure, or those with a functionally univentricular heart, respectively.2
- Very small confluent pulmonary arteries in patients below one year of age, the right and left pulmonary arteries ranging from 1 to 3 millimetres in diameter.
Criterions for decision-making and selection of patients
During the period of study, we constructed modified Blalock-Taussig shunts in 580 patients. They constituted a heterogenous group, with diverse anatomical diagnoses, well-developed subclavian arteries, but normal anatomy of the aortic arch. These patients were not included for further study. Those retained for additional study were a heterogenous cohort of symptomatic cyanotic neonates, infants, and pre-school children with complex cardiac malformations, not amenable either to primary intracardiac repair, nor to construction of a bidirectional cavopulmonary connection. These patients had particular anatomic situations contraindicating the usual technique for construction of the modified Blalock-Taussig shunt.
We proceeded to perform our modification of the modified Blalock-Taussig shunt because of the presence of disproportionately small subclavian arteries, or to avoid inserting a long polytetrafluoroethylene graft in patients with anomalies of the aortic arch and its branches, and/or the systemic veins.
Thus, there were four forces driving our criterions for selection of the patients who subsequently entered our group for further study:
- The desire to place the pulmonary end of the anastomosis onto the pulmonary trunk or its bifurcation, the right and left pulmonary arteries being hypoplastic.
- The desire to use a short segment of polytetrafluoroethylene for the graft.
- The finding of disproportionately small subclavian arteries.
- Variations in the morphology of the aortic arch and its branches, and the systemic veins, which in our judgement precluded construction of the modified Blalock-Taussig shunt in conventional fashion. The anatomic arrangements, nonetheless, represented a relative rather than an absolute contraindication to construction of the modified Blalock-Taussig shunt in standard fashion.
Our new modifications were used throughout the period of study in selected instances as illustrated in Figure 1:
- 92 patients had hypoplastic pulmonary arteries as measured using the recognized pulmonary arterial indexes of McGoon1 and Nakata.2
- 14 patients had a left aortic arch with right-sided descending thoracic aorta.
- 3 patients had left aortic arch with retroesophageal origin of an anomalous right subclavian artery
- 23 patients had a right aortic arch with right-sided descending thoracic aorta.
- 8 patients had a right aortic arch with mirror-imaged branching of the brachiocephalic arteries.
- 5 patients had a right aortic arch with retroesophageal origin of an anomalous left subclavian artery.
- 3 patients had a right aortic arch with retroesophageal origin of an anomalous left brachiocephalic artery (Fig. 2).3

Figure 2. Angiocardiogram of a patient with right aortic arch and retroesophageal anomalous left innominate artery.
- 1 patient had a right aortic arch, right descending thoracic aorta, and isolation of the left subclavian artery.
- 3 patients had a double aortic arch with a minor anterior left component.
- 22 patients had a disproportionately small left subclavian artery as compared to the desired size of the graft in those undergoing left-sided shunting procedures.
- 9 patients had disproportionately small right subclavian arteries.
- 1 patient had a right-sided heart, a right aortic arch, right-sided descending thoracic aorta, left superior caval vein, and hemiazygos continuation of the interrupted inferior caval vein (Fig. 3).

Figure 3. Intraoperative view of the centrally placed aortopulmonary shunt (S) between the ascending aorta (A) and the proximal part of the left pulmonary artery (P) (intra-extra pericardial junction). This patient had a right-sided heart, right aortic arch, right-sided descending thoracic aorta, left superior caval vein (LSVC) and hemiazygos continuation of an interrupted inferior caval vein (***) masking the origin of the great arterial branches. Due to the peculiar anatomical arrangement, a longer segment of polytetrafluoroethylene conduit had to be utilized.
We excluded all patients undergoing construction of modified Blalock-Taussig shunts as part of a rapid two-stage arterial switch operation, or conventional central aortopulmonary shunting procedures. Patients with previously placed shunts, stenosis of the pulmonary arteries at their origin, and nonconfluent pulmonary arteries, were considered unsuitable candidates for our modification of the modified Blalock-Taussig shunt, and were excluded. In this subset of patients, we constructed modified Blalock-Taussig shunts either unilaterally or bilaterally as staged palliation.
Characteristics of the patients
The patients were entered in the study protocol after informed consent had been obtained from their parents or guardians. We constructed conventional modified shunts in a total of 580 cyanotic patients between January 1996 and December 2003 at All India Institute of Medical Sciences, New Delhi, India, with a further 92 selected patients meeting the criterions for our further modification of the shunt (Table 1). Of these, 50 were male. Their age ranged from 7 days to 3.6 years, with a mean of 7.08 months, and a standard deviation of 6.16 months. Half of the patients were less than one month of age, and 10 (10.8%) were newborns less than one week of age. Their weight ranged from 2.0 to 11.0 kilograms, with a mean of 7.2 kilograms (Fig. 4). In 9 patients (9.7%), there were symptoms of dysphagia, while 7 patients (7.6%) presented with recurrent stridor and respiratory distress.
Table 1. Demographic characteristics and eventual outcome of the 92 children in whom we constructed our modification of the Blalock-Taussig shunt as an initial procedure.


Figure 4. Scattergram showing the distribution of the weights of the patients, and the size of the graft at the time of placement of the shunt.
Diagnosis was established by echocardiography in all. They were grouped into four categories (Table 1). Cardiac catheterization and angiocardiography was performed for delineation of pulmonary arterial anatomy and the laterality of the arterial duct in 11 patients, to delineate the anatomy of the aortic arch in 50 patients, and balloon atrial septostomy was performed to relieve a restrictive atrial septum in 18 patients. In 20 patients (21.7%), we performed magnetic resonance imaging to demonstrate the anatomy of the aortic arch. Isomerism of the atrial appendages was present in 17% of the patients with a functionally univentricular heart, while 39 patients (42.3%) had persistent patency of the arterial duct.
Preoperatively, 46 children with cyanotic spells with a patent arterial duct, or a closing duct less than one month of age, were started on prostaglandin E1 at doses of 0.05 to 0.1 micrograms per kilogram per minute to ensure patency of the arterial duct, and to improve the flow of blood to the lungs. In 18 infants, mechanical ventilation was required preoperatively. Among them, 9 patients were in low cardiac output, requiring dopamine. Children not responding to administration of prostaglandin, and those presenting late with cyanotic spells, were subjected to emergency procedures.
Surgical technique
The final decision to modify still further the technique of constructing the shunt was taken intra-operatively after assessment of the anatomy, the surgeon deeming this unsuitable for construction of a regular modified Blalock-Taussig shunt. Hence, in patients who underwent planned surgery, thoracotomy was the automatic choice, as the initial plan was to perform a Blalock-Taussig shunt. Concomitant division of the symptomatic vascular rings was an additional reason for approaching though a thoracotomy. The thoracotomy was right-sided in 45 patients, and left-sided in 43. In the other 4 patients, the approach was via a median sternotomy due to haemopericardium following an attempted pulmonary balloon valvoplasty. If the arterial duct was the only source of flow of blood to the lungs, the shunts were constructed opposite the side of the arterial duct, which was not ligated. In the others, the arterial duct was ligated at the time of construction of the shunt. In 16 patients, vascular rings causing symptoms were surgically corrected as a planned single-stage procedure. If the arterial duct or arterial ligament was a component of the vascular ring, it was mobilized and transected. In 3 patients, there was a double aortic arch with a dominant right component. The minor left arch in these patients was divided distal to the left subclavian artery, and the transected non-dominant end was used for the systemic-to-pulmonary arterial connection (Figs. 1C, 1D).
Our own modified shunt was constructed from the ascending aorta in 14 patients, the aortic isthmus in 29 patients, the distal aortic arch in 36 patients, the proximal descending thoracic aorta in 10 patients, the transected non-dominant end of the double aortic arch in 3, and in all instances was anastomosed to the superior portion of the intrapericardial pulmonary arteries at the bifurcation of the pulmonary trunk. The type and size of the shunt was decided during operation. This was 3.5 millimetres in 10 patients, 4 millimetres in 42 patients, and 5 millimetres in 40 patients (Fig. 4). The next important step was to test the placement of small side-biting clamps on both the aorta and pulmonary arteries. Care was taken not to occlude totally the aorta or the arterial duct if it was the sole source of supply of blood to the lungs. Heparin at a dose of one milligram per kilogram was administered intravenously. Before applying the aortic side clamp, the proposed center point of the aorta was marked with a suture. A segment of thin-walled polytetrafluoroethylene graft was selected, and the aortic end was bevelled to an angle of 45 degrees or greater like a funnel. A 4 millimetre aortic punch was used to make the aortic orifice for anastomosis. The aortic anastomosis was performed using 6-0 or 7-0 monofilament polypropylene suture. The clamp was temporarily released to check for adequacy of flow. The graft was irrigated with heparinized saline and trimmed to adequate length. The distal end of the pulmonary artery was looped using a “Silicone-elastomer vascular loop”. The pulmonary artery on that side was then clamped proximally near the bifurcation intrapericardially in an oblique fashion to ensure flow continued to the pulmonary artery on the other side either via a patent arterial duct, or through antegrade flow from the right ventricular outflow tract.
The intrapericardial portion of pulmonary arteries closest to the bifurcation was preferred for the pulmonary anastomosis because of its wider caliber, and the capacity to provide near uniform flow to both pulmonary arteries. This site was opened between stay sutures without excision. The pulmonary anastomosis was performed using 7-0 polypropylene suture. Before completion of the anastomosis, the graft was suctioned and irrigated with heparinised saline to remove any debris. Both clamps were removed. The short segment of polytetrafluoroethylene graft was never clamped.
If the approach was via a median sternotomy, the pericardium was opened only in its cephalad portion. This limited pericardiotomy adds protection on resternotomy. The tissue plane between the aorta and the pulmonary trunk was dissected to eliminate any tissue that may cause the prosthetic tube to kink. Two marker stitches were placed, one on the anteromedial surface of the aorta, and the other on the anterior surface of the pulmonary trunk. The polytetrafluoroethylene graft was beveled at each end to conform to the curvature of the arteries. The pulmonary end was sutured first in a rhomboid configuration to avoid flattening. After this, a partially occluding small vascular clamp was placed on the aorta. The graft bridged the distance between the vessels without excessive length or distortion of the pulmonary arteries. Cardiopulmonary bypass was not required in any patient.
Heparin was not reversed. A small window was routinely made at the bottom of the pericardium to drain out blood. The pericardium was loosely approximated in all cases of in which our shunts were placed in the midline. One mediastinal tube was placed.
Postoperative management
All patients received mechanical ventilatory support for 18 to 24 hours, and were extubated when haemodynamically stable. Patency of the shunt was confirmed by detection of a murmur, satisfactory arterial blood gases, and echocardiography. Dopamine in a dose of 5 micrograms per kilogram per minute was used to improve cardiac performance and to maintain optimal systolic pressures. All patients were administered digoxin, along with diuretics after extubation prior to weaning from inotropes. Heparin was continued postoperatively in a dose of 10 units per kilogram body weight per hour for 24 to 48 hours. All patients were discharged on Aspirin at 5 milligrams per kilogram body weight daily.
Second-stage repair
There were no technical problems at the time of second-stage surgery. Only mild pericardial adhesions were present. The shunt was ligated and divided in all patients undergoing second-stage repair to facilitate mobilization of the pulmonary arteries, and to avoid subsequent distortion late postoperatively.
Assessment of operative outcome
Follow-up: We followed up the 81 patients who survived the surgical procedures every 3 months in the outpatient department for cyanosis, exercise tolerance, congestive heart failure, and shunt patency. Follow-up was complete, and ranged from 1 to 101 months, yielding 305.7 patient-years of data with a mean follow-up of 45.29 months and a standard deviation of 30.06 months. Actuarial survival at 65 months was 83.5%, with a standard deviation of 0.05% (Fig. 5). Follow-up was terminated at death or second-stage palliation or biventricular repair.

Figure 5. Actuarial survival curve of patients undergoing our modification of the modified Blalock-Taussig shunt, and proceeding to a subsequent second-stage repair.
Echocardiography was employed during follow-up to evaluate the shunt and the primary cardiac lesion. Before definitive repair, 65 patients underwent cardiac catheterization and angiocardiography. The remaining 16 survivors are awaiting catheterization.
Patency of the shunt, and adequacy of palliation: We used strict criterions as suggested by Bove and co-workers.4 Criterions for unsatisfactory palliation were complete occlusion, reappearance of cyanosis with progressive decrease in arterial oxygen tension of 5 millimetres of mercury or more, progressive increase in concentration of haemoglobin of 2 grams per 100 millilitres or more, need for a second shunt, or complete non-elective repair. For purposes of statistical analysis, the length of satisfactory palliation for any given shunt was determined as that point of time when the shunt “failed” as defined above, the shunt was electively taken down due to rising pulmonary arterial pressures, or at the second-stage of repair despite adequate palliation.
Cardiac catheterization and angiography: This was performed prior to shunting in 59 patients, and prior to the second-stage of surgery in 65 patients at 12 to 18 months of follow-up. Follow-up catheterization was done to evaluate pulmonary arterial architecture, intracardiac anatomy, and to assess candidacy for second-stage operation. McGoon's ratio was calculated.1 Deformity and luminal narrowing of approximately 25 percent was considered mild. Severe distortion was defined as stenosis greater than 50%, or shunt-related isolation of the right or left pulmonary artery. We noted the extent of stenosis of the shunt, or compromise to the systemic artery used for anastomosis.
Statistical analysis
Data were analyzed with Biomedical Data Processing Statistical Software (University of California Press, Berkeley, CA) and SPSS 10.0 statistical package (SPSS Inc., Chicago, IL, USA).
Interval-related data was expressed as the mean and standard deviation, with 95% confidence intervals. Categorical data were presented as frequency distribution and percentages. Preoperative and postoperative oxygen saturation and pH measurements were compared using the Wilcoxon matched pairs signed rank test.
Both failure of the shunt and elective takedown were taken as end-points for the length of satisfactory palliation. While it is strictly correct that a shunt is no longer functioning when it has been removed during further corrective or palliative surgery, these shunts did not actually “fail”. In order to determine the actuarial probability of adequate function, according to the size of the graft, these cases were “censored” from the study group at that point, as the shunt is no longer at risk. This was done to generate the data shown in Figure 6. The log-rank test was used to analyze the differences of actuarial probability of adequate function according to the size of the graft (Fig. 6). A p value less than 0.05 was considered statistically significant. Analysis of time-related survival of the entire study group was performed using the Kaplan-Meier method (Fig. 5).

Figure 6. Actuarial probability of adequate function of the shunt according to the size of the polytetrafluoroethylene graft (PTFE).
Results
Results in the short-term
An intraoperative thrill was palpable on the pulmonary arteries in all patients. Of the patients, 5 died in hospital (5.4%) due to gastrointestinal haemorrhage in one patient, intracerebral haemorrhage in another, ventricular arrhythmias in two patients, and pulmonary infection in the final patient. The death secondary to intracerebral haemorrhage could be due to clamping of the aortic arch or its branches or postoperative heparinisation. No patient required surgical reduction of the shunt. The postoperative “room air” arterial oxygen saturations, with a mean of 87.7%, 95% confidence limits from 83.7 to 89.6%, and mean values for pH of 7.36, with 95% confidence limits from 7.34 to 7.44, were significantly higher than the preoperative mean values (oxygen saturation, 64.2%, 95% confidence limits 62.8 to 67.6%, pH 7.22, 95% with confidence limits 7.18 to 7.29), the p value being less than 0.05. This improvement in metabolic state and arterial saturation reflects the combined effects of both medical and surgical management.
Results in the long-term
An additional 6 patients (6.8%) died after discharge from hospital due to thrombosis of the shunt in 1 patient, second-stage intracardiac repair in 2 patients, sepsis in 1 patient, pulmonary infection in 1 patient, and cerebral abscess in the final patient. Of these, the shunt was patent in 5, with only one patient dying because of complications related to the shunt. In this patient, undergoing surgery at the age of 20 days for tetralogy of Fallot with pulmonary atresia, we had constructed a right-sided shunt of 4 millimetres. The patient died suddenly 10 months after operation prior to further intervention. Thrombosis of the shunt was documented at postmortem investigation.
Of the late deaths, two occurred after second-stage complete intracardiac repair of the defect. In both instances, the shunts measuring 5 millimetres in diameter were patent 12 and 16 months after implantation. Tracheopexy was performed in one patient to relieve tracheobronchial compression. There was no evidence of formation of seromas around the grafts.
Pulmonary arterial distortion: Distortion requiring correction at the time of definitive intra-cardiac repair was found in 5 patients (6.1%), all with shunts of 4 millimetres. In these 5 patients, there was narrowing and kinking of the right pulmonary artery at the site of anastomosis of the graft. In all, we disconnected the distal anastomosis, reconstructed the right pulmonary artery using autologous pericardium, and fashioning a bidirectional Glenn connection to the reconstructed right pulmonary artery.
Growth of the pulmonary arteries: The mean ratio of the diameter of right pulmonary artery to the descending thoracic aorta on cineangiogram increased from 0.52, with standard deviation of 0.04, to 1.02, with standard deviation of 0.18, this yielding a p value of less than 0.001. The mean ratio of the diameter of the left pulmonary artery to that of the descending thoracic aorta increased from 0.59, with standard deviation of 0.06, to 1.12, with standard deviation of 0.20, the p value for this difference being less than 0.002. The mean ratio of the pulmonary trunk to the descending thoracic aorta increased from 0.67, with standard deviation of 0.08, to 1.08, with standard deviation of 0.10, giving a p value of less than 0.001. The changes were achieved independent of the size of the shunt.
Thus, the centrally directed pulmonary arterial end of the prosthetic graft allowed adequate and symmetric growth of both pulmonary arteries (Fig. 7). The pulmonary arterial growth was independent of shunt size.

Figure 7. Postoperative angiocardiogram showing our modification of the modified Blalock-Taussig shunt on the right side, in which a 5 millimetre polytetrafluoroethylene (PTFE) graft was used in a 6-month-old infant with right aortic arch, tricuspid atresia, and right-sided descending thoracic aorta. Note the centrally directed pulmonary arterial end of the prosthetic graft and adequate and symmetric growth of both pulmonary arteries.
Pulmonary arterial pressure: The grafts were patent in 64 out of 65 patients at re-catheterization. In 15 patients, mean pulmonary arterial pressure was elevated, ranging from 22 to 27millimetres of mercury, with a mean of 24.5, and standard deviation of 2.02 millimetres of mercury.
Failure of the shunts, and adequacy of palliation: The shunts constructed in 60 patients permitted second or final stage palliation 12 to 18 months after the initial shunting operation. Second-stage elective intra-cardiac repairs included construction of a superior cavopulmonary connection in 15 patients, a total cavopulmonary connection in 16 patients, and biventricular repair in 29 patients. In 21 patients with patent shunts, we await the opportunity for further operation. Definitive operation was considered inappropriate in 5 patients because of low weight for age, and to allow for further growth of the pulmonary vasculature. These patients were not classified as failures, since the shunts were patent.
The overall adequacy of palliation as estimated by product-limit methods was 98%, with a standard error of 0.01, at 8 months, 91% with a standard error of 0.02 at 12 months, and 58%with a standard error of 0.08 at 18 months. Palliation was similar for patients with all sizes of shunt (log rank, p value 0.67; Fig. 6).
Discussion
Despite advances in the surgical correction of complex cyanotic diseases in infants and children, construction of systemic-to-pulmonary shunts still remains necessary, mostly as the first stage of repair.1–6 Pulmonary arterial distortion, and complications in the use of native vessels to increase the flow of blood to the lungs, led to the more frequent use of polytetrafluoroethylene shunts, either in a central position, or as a modification of the classical Blalock-Taussig shunt.6–9 The published literature shows that central aortopulmonary shunts have limited value because of the unacceptably high incidence of complications, such as thrombosis, congestive heart failure, and pulmonary arterial distortion.6–8
As yet, there is no fool-proof formula for choosing an optimal surgical approach, nor a given diameter for the shunt in any individual patient. The issue as to whether the patent arterial duct should be ligated in the setting of a duct-dependent circulation also remains controversial.4–8 Careful evaluation of the size of the patient, the aortic arch, the pulmonary arterial anatomy, and the experience of the surgeon, are all considerations when choosing the site, size, and type of shunt that will best serve an individual patient. In neonates with severely diminished flow of blood to the lungs, haemodynamic deterioration is known to occur during manipulation of the ascending aorta and pulmonary trunk, often necessitating urgent institution of cardiopulmonary bypass with its attendant risk.9, 10 Additionally, in the small infant, it is hard just to place enough aorta, and pulmonary trunk, in the partial occlusion clamp when constructing the shunt. Although an approach through a median sternotomy has been popularized for construction of central shunts by some investigators,6, 7, 9, 10 we achieved the same objectives by approaching through a thoracotomy. In the patients discussed in this report, the decision to modify still further the Blalock-Taussig shunt was made at the time of operation, once we had determined the size and relationships of the great arteries. Avoidance of near total occlusion of the aorta and its branches, maintenance of flow of blood the opposite pulmonary artery during construction of the anastomosis, division of the vascular rings, and use of the transected non-dominant end of the double aortic arch for proximal anastomosis, were the primary technical considerations for performing our modification through a thoracotomy. We are aware of four similar cases of double aortic arch, in which the transected non-dominant end of the minor arch was utilized for the systemic-to-pulmonary arterial connection.11 We approached through a left thoracotomy in all our patients with double aortic arches, in those with right aortic arch with right-sided hearts, in those with left aortic arches but right-sided descending thoracic aorta, and in a patient with hemiazygos continuation of an interrupted inferior caval vein, a left superior caval vein, and a right-sided heart. None of our patients required cardiopulmonary bypass.
Even a well-constructed shunt can thrombose, indicating that other factors may play a role. The reported incidence of thrombosis after a construction of the modified Blalock-Taussig shunt has ranged from 3.3% to 11.4%, and appears comparable to central shunts.4–10 We found that 2 of our shunts had occluded, one resulting in death, diagnosed at autopsy, and one diagnosed at catheterization. We have modified our technique so that the length of the graft is kept as short as possible, the aortic end is always bevelled, the aortotomy is fashioned with a punch, the pulmonary anastomosis is made to the intrapericardial portion of pulmonary arteries near the bifurcation, the graft is judiciously soaked in heparinised saline, intra- and postoperative systemic heparinisation is used routinely, plasma and platelets are avoided, inotropes and digoxin are used electively, and aspirin is administered daily. All of these procedures may have contributed to our good results.
Debate exists on the effect of the size of the shunt on the flow of blood to the lungs and the production of congestive heart failure. Many have reported better palliation when using grafts of 5 rather than 4 millimetres.4, 5, 9, 12, 13 We believe that conservatism is justified with regard to the diameter of the shunt. We used a conduit of 3.5 millimetres in 10 patients weighing less than 2.7 kilograms. We used shunts of 4 millimetres in all 36 patients weighing from 2.7 to 4.9 kilograms. We also prefer to place a relatively smaller conduit in patients with functionally univentricular hearts. Shunts of less that 3.5 millimetres, nonetheless, are known to prove inadequate.4, 5, 8, 9, 12, 13 We found no difference in actuarial probability of adequate function according to different sizes of the shunts. Because of concerns of pulmonary haemorrhage and congestive heart failure in newborns, we choose the size of shunt taking account of body weight, pulmonary arterial size, and ventricular morphology.
Congestive cardiac failure is undoubtedly related to volume overload induced by the shunt. Although postoperative congestive heart failure was frequent in our patients, it was easily controlled by digoxin and diuretics. We maintained one-quarter of our survivors on digoxin and diuretics for 6 to 18 months, with 30 children continuing to receive digoxin until the time of their second-stage repair. In our service, postoperative congestive heart failure uncontrolled by digoxin and diuretics, the appearance of pulmonary oedema on chest radiographs, a drop in oxygen arterial saturation with persistent metabolic acidosis, and echocardiographic evidence of over-shunting, are all indications for revision of the shunt, or clipping or ligation of the arterial duct.
The pulmonary arteries grow as a result of complex interaction of several factors, such as their initial size, the size of the shunt, and the direction of flow of blood proximal and distal to the site of anastomosis of the shunt. Results reported after palliation with either a modified or a classic shunt are conflicting.4, 5, 9, 12–16 We observed adequate growth of the pulmonary arteries, which symmetrical, and compared favourably with other reported series on central aortopulmonary shunts.6–8, 10 We presume that the shunts constructed using our modification have a likelihood of greater inflow and outflow capacity, and hence predispose to better pulmonary arterial growth. Pulmonary arterial distortion as reported previously has also varied considerably, ranging from zero to one-third in patients with a traditionally modified shunt,4, 8, 12, 13 and from zero to three-quarters of those receiving a classic shunt.1, 5 Should they occur, then because of their distal location, distorted arteries are difficult to repair. When observed in our patients, the distortion was centrally located, and was easily repaired at the second-stage operation. Our relatively low incidence of severe pulmonary arterial distortion may be related to the central location of the anastomosis, the smaller size of the grafts, and our choice of suture material.
We divided the shunt in all patients undergoing a second-stage operation to facilitate subsequent mobilization of the pulmonary arteries. There is genuine concern that the persisting short polytetrafluoroethylene connection between the aorta and the pulmonary trunk may cause later pulmonary arterial distortion. Furthermore, we believe that not dividing a central shunt placed from the descending aorta or arch to the pulmonary arteries may run the risk of eventually developing a vascular ring of sorts. Because of this, we are reluctant to leave the graft connected to the pulmonary arteries.
We recognize that our study has its limitations. The traditional modification of the classical Blalock-Taussig shunt remains the standard approach for construction of systemic-to-pulmonary arterial shunts. Only patients deemed unsuitable for construction of the shunt in traditional fashion were considered for our modification. Hence, we have not compared directly the two groups of patients. When considering adequate and symmetric growth of the pulmonary arteries, it may be better to consider our modification from the perspective of a complementary technique for central shunting, and not as a competitive one. The principal disadvantage of our technique is the invasion of pericardium, but in our experience this has not increased the morbidity or mortality in second-stage cardiac operations. Occlusion of the pulmonary trunk in the absence of an arterial duct or other source of flow of blood to the lungs also limits the use of this shunt. In these cases, we prefer the standard modification of the Blalock-Taussig shunt.









