Becker and Duchenne muscular dystrophy are the most common among muscular dystrophies, with an incidence of 1 in 18,500 and 1 in 4700 live male births, respectively.Reference Dooley, Gordon, Dodds and MacSween 1 , Reference Bushby, Thambyayah and Gardner-Medwin 2 Patients with Becker and Duchenne muscular dystrophy have a mutation in the DMD gene that leads to a deficiency or absence of dystrophin protein.Reference Moser 3 , Reference Hoffman, Brown and Kunkel 4 This results in skeletal muscle weakness and loss of ambulation, usually in the second decade of life in Duchenne muscular dystrophy and later in life in the milder Becker muscular dystrophy. Both reduced and absent dystrophin also lead to the development of cardiomyopathy, resulting in progressive left ventricular dysfunction.Reference Nigro, Comi, Politano and Bain 5 , 6
Understanding of the clinical importance of Becker and Duchenne cardiomyopathy has increased significantly over the past few decades. This is primarily due to improvements in care, which have increased life expectancy in patients with Duchenne muscular dystrophy and unmasked the cardiovascular phenotype.Reference Eagle, Baudouin, Chandler, Giddings, Bullock and Bushby 7 The preponderance of Becker and Duchenne research focuses on skeletal muscle weakness, but cardiovascular disease is now one of the leading causes of death in patients with Duchenne muscular dystrophy.Reference Bach and Martinez 8 Highlighting the effects of cardiovascular disease on inpatient morbidity, such as length of stay, cost of hospitalisation, and readmission rate, in patients with Becker and Duchenne may help improve recognition of the problems associated with cardiovascular disease. Moreover, identifying risk factors leading to increased length of stay, cost of hospitalisation, and readmission rates in this population can increase recognition and potentially improve outcomes in future admissions.
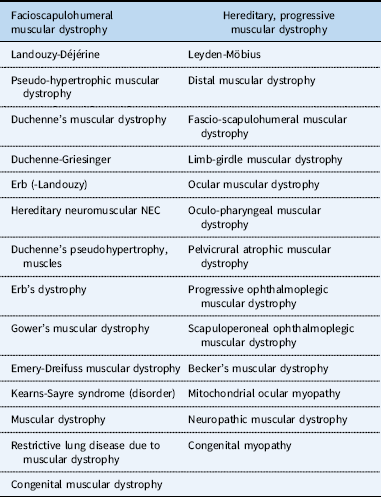
Because Becker and Duchenne muscular dystrophy are relatively rare, the true impact of cardiovascular disease can be difficult to determine. Research using large administrative databases can overcome these issues. However, using the International Classification of Disease-9 billing codes, Becker and Duchenne muscular dystrophy is coded as 359.1, a non-specific code that also encompasses multiple other neuromuscular diagnoses (Table 1). This non-specific coding can make analysis problematic. Indeed, a recent manuscript using the Pediatric Health Information System database to evaluate the effect of cardiovascular disease in Duchenne muscular dystrophy was limited by this non-specific coding.Reference Punnoose, Kaltman, Pastor, McCarter, He and Spurney 9 We hypothesised that, by creating a novel list of exclusion criteria which could be validated, patients with Becker and Duchenne muscular dystrophy could be more accurately identified within an administrative and billing database, allowing for the current study and future assessments in this patient population. To demonstrate the potential utility of this technique, we used the Pediatric Health Information System database to assess cardiovascular risk factors associated with increased length of stay, cost of hospitalisation, and 14-day readmission in patients with Becker and Duchenne muscular dystrophy.
Table 1 List of diagnoses included in International Classification of Diseases-9 code 359.1.
Materials and methods
Pediatric Health Information System database and identification algorithm
This multi-centre retrospective cohort study used administrative data from the Pediatric Health Information System database. The database contains inpatient, emergency department, ambulatory surgery, and observation encounters from 49 tertiary children’s hospitals in the United States. Data are de-identified at the time of submission and are subjected to reliability and validity checks by participating hospitals, Children’s Hospital Association (Lenexa, KS, United States of America), and Truven Health Analytics (Ann Arbor, MI, United States of America) before inclusion in the database. Patients are assigned up to 41 International Classification of Diseases-9/-10 diagnosis and procedure codes, and consistently encrypted medical record numbers allow longitudinal tracking of patients across encounters to the same hospital. The Pediatric Health Information System database represents approximately 15% of the national paediatric hospitalisations and 46.4% of children’s hospitals’ total volume.
The Institutional Review Boards of Vanderbilt University Medical Center, Nationwide Children’s Hospital, and Children’s National Medical Center approved this study. Using International Classification of Diseases-9 diagnosis codes, with conversion to International Classification of Diseases-10 codes occurring in the last quarter of 2015, an algorithm of exclusion criteria termed as the “identification algorithm” was created to improve the identification of patients with Becker and Duchenne muscular dystrophy. The effectiveness of this algorithm was evaluated by “re-identification” of patients from three sites. The electronic medical record was queried to determine the diagnosis and gender of all patients with an International Classification of Diseases-9 code of 359.1 and the diagnosis of remaining patients after the identification algorithm was applied.
The algorithm was constructed to optimise the specificity of identifying patients with Becker and Duchenne muscular dystrophy. Given the size of the Pediatric Health Information System database, investigators determined that incorrectly excluding patients with Becker and Duchenne muscular dystrophy was preferable to incorrectly including patients with an alternate diagnosis. The goal at the outset of the study was to construct an algorithm where at least 80% of remaining patients carried a diagnosis of Becker and Duchenne muscular dystrophy. All patients with the International Classification of Diseases-9 code of 359.1 were initially included. In order to eliminate errors in coding, patients with a change in primary diagnosis to a different 359.x code were excluded. A set of clinical characteristics was created to exclude neuromuscular conditions other than Becker and Duchenne muscular dystrophy. Briefly, patients with severe disease at a young age felt but unlikely to be Becker or Duchenne were eliminated, that is patients with early mortality, in ventilatory support, or cardiovascular disease were eliminated. This is because the majority of patients with Becker and Duchenne do not require gastrostomy tubes until later in life, and only 15% of patients were older than 18 in this cohort, so all patients with gastrostomy tubes were removed. Other neurologic diagnoses that could have been mistakenly coded as 359.1 were removed, as were diagnoses that came up in an initial search of 359.1 at one institution. Patients undergoing heart transplantation were not excluded as transplantation has been performed in the Becker muscular dystrophy population.
Primary analysis
The primary analysis was performed on the entire cohort of 49 hospitals after application of the identification algorithm. Discharges in patients with muscular dystrophy were identified in Pediatric Health Information System from January 1, 2003 to September 30, 2015, which were chosen based on transition from International Classification of Diseases-9 to International Classification of Diseases-10. Patients with left ventricular dysfunction and arrhythmia were identified using International Classification of Diseases-9 codes (Supplementary Table S1). Patients requiring respiratory support (Supplementary Table S1) were also identified. Discharges that occurred under 1 year of age were eliminated from the analysis as these admissions were more likely to reflect neonatal and infant hospitalisations unrelated to the diagnosis of Becker and Duchenne muscular dystrophy. The primary outcome measures included length of stay, cost of hospitalisation estimated from charges using hospital/year specific ratios of cost to charge, 14-day all-cause readmission, and in-hospital mortality.
Statistical analysis
To assess the effectiveness of the identification algorithm, the percentage of the patients with Becker and Duchenne muscular dystrophy before and after application of the algorithm was calculated for the three sites. The percentage of patients with Becker and Duchenne muscular dystrophy who were incorrectly excluded was also calculated.
For the primary analysis on the entire Pediatric Health Information System cohort, pertinent demographic information was summarised and bivariate analyses were performed to assess the relationships of each demographic and length of stay stratified by <2 days versus 2+ days using Chi-square and Wilcoxon rank-sum tests. Next, separate generalised linear mixed-effects models assuming either an exponential distribution for length of stay and cost, or a binomial distribution for 14-day readmission and mortality during the outcome were used to estimate the adjusted associations with age, encounter severity, and respiratory disease. A random intercept was used to account for correlation arising from taking repeated measurements on the same hospital. Results are summarised using the rate ratio or odds ratio with corresponding 95% confidence intervals. Statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, NC, United States of America), and a p-value <0.05 was considered statistically significant.
Results
Identification algorithm
The final identification algorithm is listed in Table 2. At the three Pediatric Health Information System sites, we performed a detailed assessment of our algorithm by re-identifying the 289 patients with an International Classification of Diseases-9 of 359.1 and determining true diagnosis using the local electronic medical record. Of the 289 patients, 55% (158) had a diagnosis of Becker and Duchenne muscular dystrophy before the application of the identification algorithm. Excluding female patients increased this to 68% (158 of 233). Applying the identification algorithm increased the accuracy of correct Becker and Duchenne muscular dystrophy diagnosis to 77% (131 of 170) by excluding 27 patients with Becker and Duchenne muscular dystrophy (17%) and 91 patients without Becker and Duchenne muscular dystrophy – 70% of non-Becker and non-Duchenne muscular dystrophy patients. Of note, two sites provided a breakdown of Becker muscular dystrophy versus Duchenne muscular dystrophy, and only 5% of included patients at those sites had Becker muscular dystrophy. We hypothesise that this preponderance of Duchenne muscular dystrophy is due to the relatively higher frequency in the general population, the earlier onset of cardiomyopathy, and the lower frequency of admission in children with Becker muscular dystrophy.
Table 2 Identification algorithm.

* Gastrostomy tube (G-tube)
** Continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP)
*** Automatic implantable cardioverter-defibrillator (AICD)
Demographics
In the entire Pediatric Health Information System cohort, a total of 3430 unique patients with 13,189 discharges from 49 hospitals were identified with an International Classification of Diseases-9 of 359.1. After application of the identification algorithm, 1916 patients and 4014 discharges remained.
The majority of discharges (89.5%) were to home, 4% were discharged to a skilled facility, 3.1% to home health service, and 2.2% (90 discharges) died in the hospital (4.7% of patients). As expected, neuromuscular and cardiovascular codes were the most common, with 90% of discharges having at least one neuromuscular code and 30% having at least one cardiovascular code. In addition, 22% had a code for congenital or genetic defects, 21% had a code suggesting technology dependence, and 7% had a code denoting respiratory disease. A total of 26% of discharges had a diagnosis of left ventricular dysfunction, 8% arrhythmia, and 21% respiratory disease. Further demographic data are reported in Table 3.
Table 3 Demographics.

ICD=International Classification of Diseases; IQR=interquartile range
Primary analysis
Bivariate analysis for the predictors left ventricular dysfunction, arrhythmia, and respiratory disease for the outcomes of length of stay, cost of hospitalisation, 14-day readmission, and mortality are shown in Tables 4–7. We also considered adjusted models controlling for age, encounter severity, respiratory disease, and hospital. The adjusted association of predictors with mortality was not estimated because there were too few events to support a multi-variable model. However, for length of stay we found significant adjusted association with left ventricular dysfunction (rate ratio=1.4, 95% confidence interval [1.3, 1.5], p<0.001), arrhythmia (rate ratio=1.2, 95% confidence interval [1.1, 1.4], p=0.004), and respiratory disease (rate ratio=1.6, 95% confidence interval [1.5, 1.8], p<0.001) (Tables 8–10). We also found significant adjusted association for cost of hospitalisation with left ventricular dysfunction (rate ratio=1.2, 95% confidence interval [1.1, 1.3], p<0.001), arrhythmia (rate ratio=1.4, 95% confidence interval [1.2, 1.6], p<0.001), and respiratory disease (rate ratio=1.4, 95% confidence interval [1.3, 1.5], p<0.001). Becker and Duchenne muscular dystrophy patients with respiratory disease had decreased rates of 14-day readmission (odds ratio=0.6, 95% confidence interval [0.3, 1.0], p=0.045), while left ventricular dysfunction (odds ratio=1.0, 95% confidence interval [0.7, 1.3], p=0.780) and arrhythmia (odds ratio=1.5, 95% confidence interval [0.9, 2.5], p=0.128) were not significantly associated with readmission. The combination of arrhythmia and left ventricular dysfunction (cardiovascular disease) demonstrated similar effects for hospitalisation cost and length of stay (Supplementary Table S2).
Table 4 Bivariate analysis for decreased left ventricular (LV) function.

* Interquartile range
Table 5 Bivariate analysis for arrhythmia.

Table 6 Bivariate analysis for respiratory disease.

Table 7 Number of discharges with each combination of exposure.

Table 8 Generalized linear mixed-effects model with LOS as outcome measure.

LOS=length of stay; LV=left ventricular
Table 9 Generalised linear mixed-effects model with cost of hospitalisation as outcome measure.

LV=left ventricular
Table 10 Generalised estimating equation with 14-day readmission as outcome measure.

LV=left ventricular
Discussion
These results demonstrate that the application of our identification algorithm confirmed by direct validation significantly increases the specificity for Becker and Duchenne muscular dystrophy in the Pediatric Health Information System database. They also demonstrate that Left ventricular dysfunction, arrhythmia, and respiratory disease play significant, independent roles in predicting inpatient morbidity and, in the bivariate model, mortality. Given the rarity of Becker and Duchenne muscular dystrophy, a method that allows researchers to leverage large databases is critical to improving outcomes. Analysis of the Pediatric Health Information System database may help identify areas of improvement that could lead to a decrease in the costs of hospitalisation for patients with Becker and Duchenne muscular dystrophy. Moreover, these data can serve as a baseline appraisal, allowing for the assessment of future shifts in outcome resulting from modifications in therapies or changes to standards of care in Becker and Duchenne muscular dystrophy.
The International Classification of Diseases-10 billing code that includes Becker and Duchenne muscular dystrophy also includes many other diagnoses. Fortunately, this will be rectified in October of 2018 with a new International Classification of Diseases-10 code. However, given that this manuscript used over 12 years of data from the Pediatric Health Information System, it will take an extended period of time for that change to be relevant. To allow for future analyses, we have translated our exclusion criteria to ICD-10 diagnostic codes (Supplementary Table S3). The algorithm reported here will remain critical for future analyses and will facilitate analysis of historical trends. In addition, similar methods can be used to evaluate morbidity and mortality due to other forms of muscular dystrophy coded as 359.1.
These data further emphasise the importance of cardiovascular disease in Becker and Duchenne muscular dystrophy. It is notable that 30% of the patients in this analysis had at least one cardiovascular code, while only 7% had a code for respiratory disease. Cardiovascular disease has become increasingly recognised as a major contributor of mortality.Reference Bach and Martinez 8 , Reference Connuck, Sleeper and Colan 10 Punnoose et al recently evaluated the association between cardiovascular disease and morbidity and mortality in the Pediatric Health Information System database, though their analysis was limited by the poor specificity of International Classification of Diseases-9 coding for Duchenne muscular dystrophy.Reference Punnoose, Kaltman, Pastor, McCarter, He and Spurney 9 Their study also demonstrated that ventricular tachycardia and heart failure, as well as chronic ventilator use, were risk factors for the combined outcome of cardiac arrest or death. Their exclusions likely improved specificity for Duchenne muscular dystrophy but are less extensive than the algorithm we present here, and they were unable to directly validate the effectiveness of their exclusion criteria. The prevalence of cardiomyopathy increases significantly as Becker and Duchenne muscular dystrophy patients age, with previous studies suggesting that >60% of patients over 18 years of age have cardiomyopathy.Reference Nigro, Comi, Politano and Bain 5 , Reference Spurney, Shimizu and Morgenroth 11 Unfortunately, while official recommendations state that echocardiographic screening should begin by 6 years of age, Spurney et al demonstrated that a surprisingly large portion of Duchenne muscular dystrophy patients had not undergone echocardiography by 10 years of age.Reference Spurney, Shimizu and Morgenroth 11 Moreover, the study revealed that over half of Duchenne muscular dystrophy patients with a diagnosis of cardiomyopathy were not prescribed appropriate anticongestive therapy. Taken together, these data suggest that the focus of care in Becker and Duchenne muscular dystrophy must shift toward earlier detection and prevention of cardiovascular disease. Raising awareness of the risks of cardiovascular disease in Becker and Duchenne muscular dystrophy patients admitted to the hospital may lead to earlier recognition and more aggressive therapy, eventually improving outcomes.
Our data confirm that respiratory disease continues to play a significant role in inpatient morbidity and mortality in patients with Becker and Duchenne muscular dystrophy. Multiple authors have reported the improved life expectancy associated with ventilation in Duchenne muscular dystrophy.Reference Eagle, Baudouin, Chandler, Giddings, Bullock and Bushby 7 , Reference Bach and Martinez 8 , Reference Eagle, Bourke and Bullock 12 While survival has increased significantly with these interventions, respiratory disease of any kind, including acute respiratory complications and the need for chronic ventilation, significantly increased the odds of a longer length of stay and higher cost of hospitalisation. Moreover, the complex interactions between cardiovascular and respiratory disease likely play an important role in Becker and Duchenne muscular dystrophy inpatient morbidity.
Multiple reports have demonstrated the increased cost of both Duchenne and Becker muscular dystrophy compared with the general population. Thayer et al demonstrated a 10-fold increase in costs in a small cohort of 75 Duchenne muscular dystrophy patients by analysing claims from a single health plan.Reference Thayer, Bell and McDonald 13 Surveys of families in multiple countries have demonstrated increases in both healthcare costs and out of pocket costs to families of Becker and Duchenne muscular dystrophy patients.Reference Teoh, Geelhoed, Bayley, Leonard and Laing 14 – Reference Landfeldt, Lindgren and Bell 16 In general, costs increase with increasing age/progression of disease.Reference Teoh, Geelhoed, Bayley, Leonard and Laing 14 , Reference Schreiber-Katz, Klug and Thiele 15 Costs have also been shown to be increased in Duchenne muscular dystrophy patients with tracheostomy compared to those on continuous non-invasive ventilation.Reference Bach, Tran and Durante 17 However, only our data and that of Punnoose have stratified costs based on cardiovascular disease.Reference Punnoose, Kaltman, Pastor, McCarter, He and Spurney 9 Awareness of the increased cost associated with both arrhythmia and left ventricular dysfunction could allow for improvement in Becker and Duchenne muscular dystrophy costs with earlier and more aggressive detection and therapy. Not surprisingly, our data also demonstrated significantly increased length of stay and cost of hospitalisation with advancing age in Becker and Duchenne muscular dystrophy, even when correcting for arrhythmia, left ventricular dysfunction, and respiratory disease.
Limitations
This study does have inherent limitations. As with any large database, data could be incomplete, incorrect, or missing. The Pediatric Health Information System does not capture events that occur outside a hospital encounter and does not include physician fees as part of the cost analysis. In addition, the Pediatric Health Information System only captures events that occur in children’s hospitals. Specific to this study, our algorithm prioritised identification of Becker and Duchenne muscular dystrophy patients with the knowledge that some patients would be incorrectly excluded. However, re-identification demonstrated that 17% of Becker and Duchenne muscular dystrophy patients were incorrectly excluded, while 70% of non-Becker and Duchenne muscular dystrophy were excluded. The algorithm we present to better identify patients with Becker and Duchenne muscular dystrophy does still include patients without Becker and Duchenne muscular dystrophy, but this number is significantly lower after our exclusion criteria and we feel the advantages of using larger numbers for this and future studies will outweigh this limitation. We also evaluated an algorithm that increased the identification of patients with Becker and Duchenne muscular dystrophy to 79% (Supplementary Table S4), but this algorithm excluded a much larger number of patients with Becker’s and Duchenne’s (35%), so the less aggressive algorithm was used for the final analysis. Of note, the results of the primary analysis were not significantly different between algorithms.
Conclusions
We present an algorithm for improving identification of patients with Becker and Duchenne muscular dystrophy using an administrative database. Initial analysis demonstrated a large number of Becker and Duchenne muscular dystrophy patients with cardiovascular codes and increased cost and length of stay in patients with both arrhythmia and left ventricular dysfunction. This algorithm can be used to analyse other outcomes of interest in patients with Becker and Duchenne muscular dystrophy and can also serve as a baseline to assess changes in therapy over time.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951118002226
Acknowledgements
None.
Financial Support
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL123938 (Bethesda, MD) (Soslow). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This project was supported by the Fighting Duchenne Foundation and the Fight DMD/Jonah & Emory Discovery Grant (Nashville, TN) (Markham).The sponsors and funders had no role in the design and conduct of the study or in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation Belmont Report and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees. The Institutional Review Boards of Vanderbilt University Medical Center, Nationwide Children’s Hospital, and Children’s National Medical Center approved this study.












