Traditionally, congenital cardiac ventricular outpouchings are classified as either aneurysms or diverticula based on histological and morphological characteristics. Aneurysms’ wall is thin and fibrous with little to absent myocardium, while diverticula have thick wall that comprises all three layers. Aneurysms therefore are not contractile and expand paradoxically during systole. On the other hand, diverticula contract normally during systole. Finally, aneurysms connect with the ventricular cavity via a wide neck as opposed to diverticulaʼs narrow neck connection.Reference Hornberger, Allan and Sharland1 However, clear distinction between these two entities remains difficult given some histological and morphological overlap.Reference Williams, Collardey, Treadwell and Owens2, Reference Prefumo, Bhide, Thilaganathan and Carvalho3
Case report
A baby boy was born at 34 weeks’ gestation to a 32-year-old female. Pregnancy was complicated by poor prenatal care, maternal chronic hypertension, pre-eclampsia diagnosed at 31 weeks’ gestation, and suspected CHD. At 32 weeks’ gestation, the anatomy scan was suspicious for Ebsteinʼs anomaly of the tricuspid valve. Subsequently, a foetal echocardiogram revealed an aneurysm in the free wall of the right ventricle near the apex. The aneurysm had a maximal diameter of 8 mm with the neck measuring 3.5 mm at end systole. There were no other intracardiac abnormalities or pericardial effusion. Biventricular systolic function was normal. Delivery was induced due to severity of maternal pre-eclampsia. APGAR scores were 3, 7, and 9 at 1, 5, and 10 minutes after birth with the neonate needing positive pressure ventilation for respiratory distress secondary to respiratory distress syndrome. The physical examination was otherwise normal. A post-natal echocardiogram was done on day 1of life, which confirmed a right ventricular aneurysm at the anteroinferior wall of the right ventricle. Interestingly, this aneurysm had features suggestive of a pseudoaneurysm: thin-walled cavity with loss of myocardial integrity, in communication with the pericardium and exhibiting paradoxical motion noticed with the colour flow pattern within the cavity, and communicating with the right ventricle cavity via a narrow neck. Moreover, there was a sharp transition with ragged edges between the ventricular cavity and the outpouching. These features are seen in Figure 1 as well as in Supplementary videos 1 and 2. The dimensions of this pseudoaneurysm were similar to those measured prenatally. The remainder of the echocardiogram was normal without pericardial effusion. No thrombus was seen either inside the pseudoaneurysm cavity or in the pericardial space. The electrocardiogram and coagulopathy panel were both normal. The infant was discharged at day 21 of life. At last follow-up at 2 months of age, he did not have signs or symptoms attributable to the cardiovascular system. The pseudoaneurysm remained stable in size.
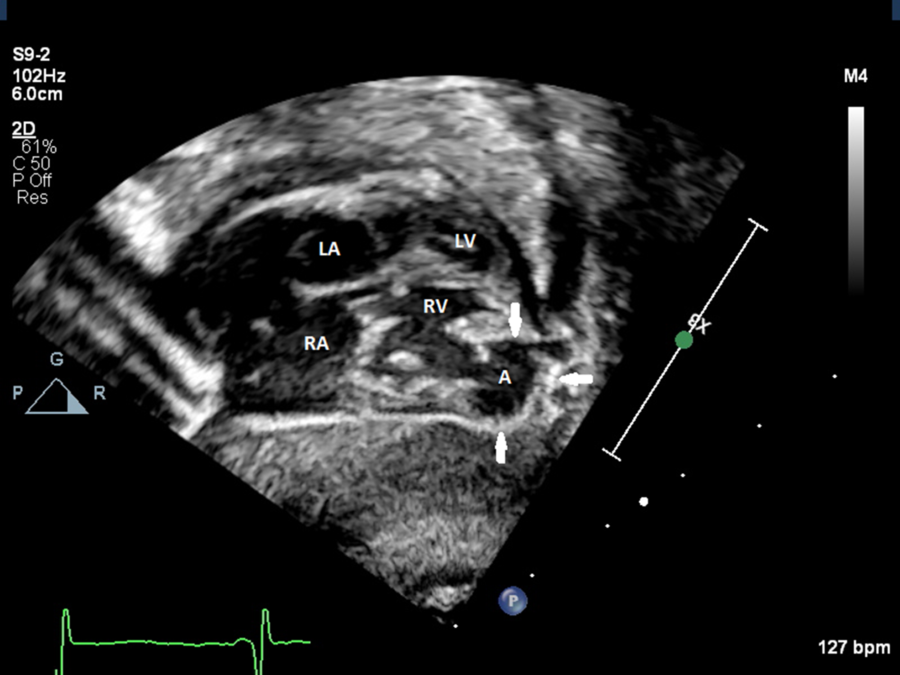
Figure 1. Subcostal frontal view using two-dimensional transthoracic echocardiogram showing the pseudoaneurysm at the free wall of RV near the apex. A = aneurysm; LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle.
Discussion
Cardiac pseudoaneurysms are defined as any rupture in the wall of the myocardium that is contained by pericardium, thrombus, or adhesions.Reference Yeo, Malouf, Oh and Seward4,Reference Dachman, Spindola-Franco and Solomon5 Ventricular pseudoaneurysms, particularly ones located in the right ventricle, are even more rare than true aneurysms or diverticula with all of the cases reported in the literature occurring as a result to cardiac surgery, endomyocardial biopsy, device lead extraction, and penetrating chest wall trauma, lipoma, infectious causes, and myocardial infarction.Reference Sakaguchi, Akagi, Totsugawa, Tamura and Hiraoka6 Pseudoaneurysms differ from aneurysms in that they connect to the ventricle via a narrow neck, their wall completely lacks myocardium, and they have a high risk to rupture.Reference Lippmann, Wiley, Freeman, Abicht and Nath7 Established echocardiographic features of pseudoaneurysms include the neckʼs diameter at end systole is less than half of the maximal diameter of the outpouching along with aliasing and bidirectional flow in and out of the outpouchingʼs cavity visualised by colour flow and spectral Doppler; spontaneous echogenic contrast and thrombus may be visualised in the pericardial space.Reference Sutherland, Smyllie and Roelandt8
Our case had echocardiographic features of a pseudoaneurysm since its wall consisted of a thinned visceral pericardium with evidence of loss of myocardial wall integrity. Moreover, the pseudoaneurysm exhibited paradoxical motion in comparison to the primary ventricular cavity with to-and-fro flow from its cavity. Finally, the pseudoaneurysm communicated with the right ventricle via a narrow neck whose diameter was less than half of the maximal aneurysmal diameter. Interestingly, we could observe a clear transition with ragged edges between the wall of the main cavity and the wall of the outpouching reminiscent of a break in the myocardial layer. We speculate that this pseudoaneurysm is a result from a congenital insult in utero leading to dehiscence of the myocardial wall.Reference Amplatz and Moller9
Due to their rarity, the management of pseudoaneurysms is guided by limited experience. While the primary goal is to avoid further rupture, the specific approaches remain highly variable since the risk of rupture appears to be associated with many factors such as the location, the nature of previous pericardial adhesions, and the chance of spontaneous obliteration.Reference Lippmann, Wiley, Freeman, Abicht and Nath7 However, in general, right ventricular pseudoaneurysms appear to be less likely to further rupture. Therefore, besides surgical resection, it could be reasonable to manage these pseudoaneurysms conservatively since the chances of rupture are extremely low in right ventricular pseudoaneurysm. More prospective studies are needed to assess 1) time course from detection to rupture and 2) risk factors for rupture.
So far, our patientʼs pseudoaneurysm has remained stable in size, and it has not caused any signs or symptoms. However, serial and close follow-up is needed.
Conclusion
An isolated congenital right ventricular aneurysm could present with characteristics of pseudoaneurysms clearly visualised on echocardiography. Correct diagnosis of pseudoaneurysms is important due to their possible different natural history and management from those of aneurysms and diverticula.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951120000633
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.



