Aneurysmal dilatation of the ductus arteriosus is a rare but potentially fatal abnormality. It is often congenital. However, acquired cases have also been reported, as a complication of surgical closure or as a sequelae of infective endarteritis of a patent ductus. Congenital ductal aneurysm can be further subdivided into two groups: infantile and adult onset types. We report a case of congenital ductal aneurysm in whom there was also intestinal atresia, diagnosed as a neonate, who was later found to have a novel mutation in MYH11.
Case report
Our patient was born full term to a 29-year-old mother, a product of non-consanguineous parents. Prenatal fetal ultrasound showed polyhydramnios along with distended fetal bowel. Family history was negative for congenital anomalies or genetic disorders. Physical examination at birth demonstrated a neonate with no respiratory distress and normal vital signs. Abdominal examination revealed a mildly distended abdomen with no tenderness and no organomegaly. Abdominal X-ray and a subsequent upper gastrointestinal barium series demonstrated findings consistent with duodenal stenosis (second part) along with proximal jejunal atresia.
Cardiology evaluation was requested on day one of life, to rule out associated congenital heart disease and obtain clearance for surgical repair of the gastrointestinal anomaly. There were normal heard sounds and no murmurs. An ECG performed was normal. An echocardiogram showed normal intracardiac anatomy and ventricular function. However, there was aneurysmal severe fusiform dilatation of a left-sided ductus arteriosus. The aortic end of the ductus measured 5 mm in diameter, the mid portion 9 mm, and the pulmonary artery end 1 mm. The flow across the patent ductus arteriosus was restrictive at the pulmonary artery end, with continuous left-to-right shunt by color Doppler (Fig 1, Supplementary video 1). 2D imaging showed spontaneous contrast with a slow swirling flow in the ductal aneurysm. However, there was no thrombus seen. The infant had no dysmorphic physical features or evidence of connective tissue disease. Ultrasound evaluation of the kidneys and bladder was within normal limits. Based on the above evaluation, the neonate was cleared for the intestinal surgery. A genetic analysis done in view of multiple anomalies demonstrated a normal 46, XX karyotype, and a normal chromosomal microarray with no deletions or duplications.
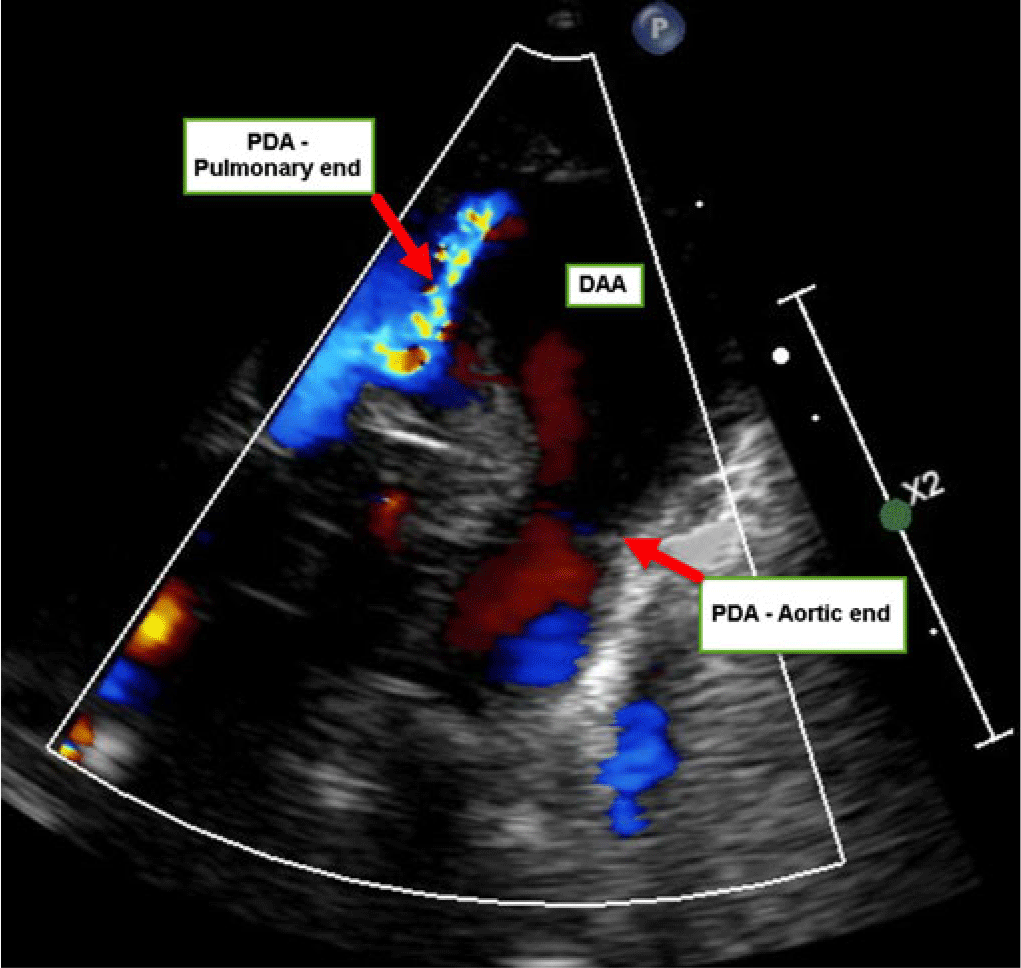
Figure 1. Echocardiogram parasternal short axis view with color Doppler showing the aneurysmal ductus. DAA = ductus arteriosus aneurysm; PDA = patent ductus arteriosus.
At 2 days of age, the infant underwent exploratory laparotomy which demonstrated type IIIB jejuno-intestinal atresia, characterised by atresia of the jejunum with the absence of distal superior mesenteric artery and coiling of distal small bowel like an “apple peel.” A Ladd’s procedure along with resection of the atretic portion and side-to-side jejunal anastomosis were performed. The neonate did well with no post-operative complications. Repeat echocardiogram performed at 5 days of age demonstrated spontaneous closure of the ductus arteriosus due to complete thrombosis of the aneurysmal portion of the ductus, with no evidence of thrombus extension into the pulmonary artery or the aortic arch (Fig 2, Supplementary video 2).

Figure 2. Echocardiogram parasternal short axis view showing thrombosed ductal aneurysm. DAA = ductus arteriosus aneurysm; MPA = main pulmonary artery.
In view of a known association of ductal aneurysm with genetic and connective tissue disorders, a DNA sequence analysis and exonic deletion/duplication testing of the 27 genes implicated in aortopathy (Invitae® Aortopathy Comprehensive Panel) were performed. This identified a novel heterozygous mutation (c.4366A>C: p. Lys1456Gln) in MYH11 gene, not previously reported. Repeat echocardiogram at 3 months of age demonstrated no evidence of patent ductus with complete resolution of the ductal aneurysm and normal aortic dimensions.
Discussion
Congenital ductus arteriosus aneurysm is a saccular or fusiform dilatation of a persistent ductus arteriosus. These aneurysms likely develop in the third trimester perhaps due to abnormal intimal cushion formation or elastin expression. Ductal aneurysm is most commonly diagnosed in children under 2 months of age,Reference Dyamenahalli, Smallhorn and Geva1,Reference Lund, Hansen, Brocks, Jensen and Jacobsen2 with only a few cases reported in adulthood.Reference Mitchell, Seifert, Miller, Jamieson and Shumway3 Earlier reports on incidence of ductal aneurysms included only patients with incidental diagnosis during work up of abnormal chest radiography or during thoracotomy performed for other pathologies with a reported incidence of 0.8%. A prospective study in 2002 by Jan et al, evaluating 548 term newborns with screening echocardiography, concluded a much higher incidence of 8.8%.Reference Jan, Hwang, Fu, Chai and Chi4 A retrospective study by Dyamenahalli et al, evaluating 200 consecutive third-trimester fetal ultrasounds, suggested an incidence of aneurysmal ductus arteriosus of 1.5%.Reference Dyamenahalli, Smallhorn and Geva1
Ductal aneurysm has been described in the literature as a possible normal variant occurring as an isolated anomaly, but it may also be associated with connective tissue disorders and genetic syndromes. In the literature, there are several reports of aneurysmal ductus in association with genetic syndromes such as Marfan,Reference Crisfield5 Ehler-Danlos,Reference Chang, Chang and Sheih6 Smith–Lemli–Opitz, Trisomy 13, and Trisomy 18.Reference Dyamenahalli, Smallhorn and Geva1
Mutations in smooth muscle contractile elements, including alpha smooth muscle actin encoded by ACTA2 gene and smooth muscle myosin encoded by MYH11 gene, have been implicated in ductus arteriosus aneurysm. The proposed mechanism for persistence and aneurysmal dilatation of ductus arteriosus is failure of the ductus to constrict after birth. ACTA2 mutation has been recently identified as a major cause of familial thoracic aortic aneurysm and ductus arteriosus aneurysm.Reference Logeswaran, Friedburg and Hofmann7 Several MYH11 mutations have been implicated in adults with thoracic aortic aneurysms and patent ductus arteriosus.Reference Zhu, Vranckx and Van Kien8 A dominant causal variant in MYH11 associated with smooth muscle dysfunction has been reported recently in families with chronic intestinal pseudoobstruction.Reference Dong, Baldwin and Choi9 The MYH11 variant in our patient is a novel mutation not previously described. This mutation could be a pathogenic one in our patient due to the existence of two concomitant smooth muscles disorders: ductal aneurysm and intestinal atresia. The authors believe that the patient will likely need close follow up to monitor for potential late development of thoracic aortic aneurysm.
The pathogenesis of congenital ductal aneurysm remains ill defined, although several theories have been postulated.Reference Logeswaran, Friedburg and Hofmann7 These include (a) delayed closure of the aortic end of the ductus, resulting in exposure of the ductal wall to systemic arterial pressure, (b) necrosis and mucoid degeneration of the media of the ductus, resulting in weakness of the wall, (c) congenital weakening of the ductal wall from defective elastin, resulting in abnormal intimal cushion formation, and (d) intrauterine constriction of the ductus close to the pulmonary end with post-stenotic dilation. In our patient, the likely mechanism of aneurysmal ductus is the failure to constrict after birth from defective smooth muscle myosin.
A majority of ductal aneurysms spontaneously regress without complications.Reference Jan, Hwang, Fu, Chai and Chi4 Spontaneous resolution of the ductal aneurysm is possible by one of two mechanisms: most due to progressive constriction and the remaining from thrombus formation.Reference Logeswaran, Friedburg and Hofmann7 However, severe and life-threatening complications have been reported. These include thromboembolic extension into the main pulmonary artery or the aorta, spontaneous rupture, erosion or compression of adjacent structures, and infection. Based on a review of literature by Lund et al, the complication rate is more than 30% in children less than 2 months and it increases to 60% beyond 2 months of age.Reference Lund, Hansen, Brocks, Jensen and Jacobsen2 However, these complication rates are based on autopsy findings, and therefore the true risk may be lower.
Close follow-up with echocardiogram is recommended to assess for spontaneous resolution of ductal aneurysms. It has been suggested that surgical resection of a large ductal aneurysm may be considered if there is (a) patent ductus with aneurysm beyond the neonatal period; (b) associated connective tissue disease; (c) evidence of thrombus extension into adjacent vessels or thromboembolism; or (d) significant compression of adjacent vessels.Reference Hornberger10 Currently, there are no clear guidelines regarding indications for use of anticoagulation in ductal aneurysm, and this has to be decided on a case-by-case basis. Close follow-up is recommended in patients who had large ductal aneurysms, if there is an underlying genetic disorder. In our patient, although large ductal aneurysm has spontaneously resolved by thrombus formation, without any complications, the presence of a novel MYH11 mutation warrants closer follow-up in the future, as there is a potential risk of development of thoracic aortic aneurysm and dissection.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951119003287
Acknowledgements
The authors thank the patient’s family for their participation in this reporting.
Financial Support
This manuscript received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.




