Persistent patent ductus arteriosus is a congenital cardiac anomaly that results from failure of the ductus arteriosus to close spontaneously in the first 24 to 48 hours of life. Indications for repair include chamber enlargement, pulmonary artery hypertension, presence of net left-to-right shunting, and prior endarteritis.Reference Warnes, Williams and Bashore1 Although the traditional treatment for persistent patent ductus arteriosus is ligation by thoracotomy, alternative approaches include transcatheter device closure and minimally invasive video-assisted thoracoscopic surgery. Multiple studies in the literature support the safety and efficacy of thoracoscopic surgery, with complication rates of 0.75–5% and closure success rates of 98.2–99.1%.Reference Laborde2–Reference Nezafati, Soltani, Mottaghi, Horri and Nezafati4 In addition, comparison studies of thoracoscopy versus thoracotomy have thus far demonstrated no significant global differences in safety and efficacy.Reference Kennedy, Snyder, Ashcraft and Manning5–Reference Stankowski, Aboul-Hassan, Marczak, Szymanska, Augustyn and Cichon9
Nevertheless, the data comparing the two methods remain sparse and conflicted on multiple important technical indices. Some studies have indicated that thoracoscopic surgery is associated with shorter hospital staysReference Vanamo, Berg, Kokki and Tikanoja6,Reference Esfahanizadeh, Aghaee Meybodi and Sepehri Shamloo8 , reduced operative timesReference Vanamo, Berg, Kokki and Tikanoja6,Reference Chen, Weng and Chen7 , and fewer post-operative complicationsReference Chen, Weng and Chen7,Reference Stankowski, Aboul-Hassan, Marczak, Szymanska, Augustyn and Cichon9 , while others have described either neutral or opposing findings.Reference Vanamo, Berg, Kokki and Tikanoja6,Reference Chen, Weng and Chen7,Reference Stankowski, Aboul-Hassan, Marczak, Szymanska, Augustyn and Cichon9 Some studies have also focused entirely on neonate and infant patient populations, for which outcomes may be confounded by co-morbidities or clinical status requiring urgent ductus closure, or have included predominantly low-birth-weight infants.Reference Chen, Weng and Chen7,Reference Stankowski, Aboul-Hassan, Marczak, Szymanska, Augustyn and Cichon9 Collectively, the studies that comprise the current literature are derived from single-centre experiences in disparate countries, with the most recent United States data having been published more than two decades ago. The purpose of this study was to compare outcomes between the two procedures for patients presenting for elective patent ductus arteriosus closure with the goal of closing the gap in the literature.
Materials and methods
A retrospective chart review was performed on patients who underwent elective patent ductus arteriosus ligation, either by thoracotomy or video-assisted thoracoscopic surgery, at Boston Children’s Hospital from January 2000 to December 2017. To capture patients undergoing elective closure only, exclusionary criteria included weight at surgery <3.3 kg as a surrogate for premature patients with persistently low birth weights whose length of stays are heavily influenced by other co-morbidities. Other exclusions comprised co-morbid cardiac defects requiring procedural intervention, and the presence of co-morbidities requiring prolonged hospitalisation or ICU care. This study was approved by the Institutional Review Board of Boston Children’s Hospital.
Demographic indicators included gender, age, preterm status, weight at surgery, ductus anatomy, and co-morbid congenital anomalies (cardiac, respiratory, gastrointestinal, neurologic, and syndromic). Indications for ligation were also included: left ventricle dilation, dependence on diuretics (documented use prior to surgery as a surrogate for heart failure), echocardiographic evidence of elevated pulmonary artery pressure, and failure to thrive as documented in a physician note. Patients who had failed prior catheterisation were also noted.
For the thoracotomy approach, the standard latissimus-sparing posterolateral thoracotomy technique was utilised. For the video-assisted thoracoscopic surgery approach, patients were positioned in the right lateral decubitus position. Four incisions were made, three along the fifth interspace and one under the axilla in the periscapular region. A 30-degree-angled scope was used for visualisation. A fan retractor was used to retract the lungs and expose the superior posterior mediastinum. The pleura overlying the aorta were opened, and blunt dissection was carried out until visualisation of the ductus arteriosus was achieved. Care was taken to avoid injury to the recurrent laryngeal nerve. Standard titanium or implantable clips were used to perform ligation of the ductus. In recent years, Yasargil implantable aneurysm clips (Aesculap Inc, Center Valley, Pennsylvania, United States of America) were utilised (Fig 1). The advantages of this clip include a blunt tip compared to traditional clips, and ease of removal and repositioning. In most patients, transoesophageal echocardiogram was performed prior to chest closure to confirm lack of flow through the patent ductus arteriosus.

Figure 1. Post-operative supine radiograph of Yasargil clip deployed around ductus arteriosus.
Comparison between the thoracoscopy and thoracotomy cohorts was done on an intention-to-treat basis. Post-surgery follow-up time was calculated as time elapsed between surgery and the most recent documented encounter in the medical record. Short-term outcomes that were assessed included the following technical indices: operative time, length of hospital stay, length of ICU stay, residual patency, and conversion. Operative complications included pneumothorax, pleural effusion or chylothorax requiring chest tube intervention or re-operation, isolated chest tube placement, transfusion, infection, and vocal cord injury as documented by laryngoscopy. Long-term outcomes were obtained from documented patient notes at follow-up and included residual patency, re-operation, vocal cord injury, and mortality.
All statistical analyses were performed using IBM SPSS Statistics (Armonk, New York, USA). Chi-square and Fisher’s exact tests were used for categorical variables. Student t-test and Wilcoxon rank sum test were used for continuous variables. Propensity score matching was done to balance the thoracoscopy and thoracotomy groups on confounders potentially associated with both the exposure and outcomes, namely age at surgery, weight at surgery, dependence on diuretics, and preterm birth. Thoracotomy patients were matched to up to four thoracoscopic patients. Matching was performed without replacement using greedy matching and a calliper width of 0.01. Propensity scores were also used as inverse probability weights in regression modelling to compare thoracoscopy and thoracotomy patients on continuous outcomes. Results were considered statistically significant for two-tailed p < .05.
Results
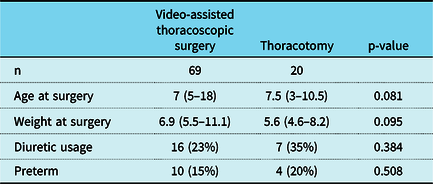
Between 2000 and 2017, 173 patients underwent persistent patent ductus arteriosus ligation and met specific inclusion and exclusion criteria. The surgical approach included video-assisted thoracoscopic surgery in 127 and thoracotomy in 46. Demographic and pre-operative information for the entire cohort are summarised in Table 1. Median age and weight at surgery were significantly higher in thoracoscopy versus thoracotomy patients (10 months versus 4 months and 7.7 kg versus 4.7 kg respectively; p < 0.001). There were also fewer preterm (9% thoracoscopy versus 28% thoracotomy; p < 0.001) and diuretic-dependent (17% thoracoscopy versus 46% thoracotomy; p < 0.001) patients. In order to account for differences in patient characteristics, propensity matching by age and weight at surgery, dependence on diuretics, and preterm status was performed. Subsequently, 69 patients remained in the thoracoscopy cohort and 20 in the thoracotomy cohort (Table 2).
Table 1. Patient characteristics (n = 173)

Abbreviations: GI = Gastrointestinal; IQR = interquartile range; LV = Left Ventricular; NA = Not Applicable; PA = Pulmonary arterial; VATS = video-assisted thoracoscopic surgery.
* Included patent foramen ovale, atrial septal defects, ventricular septal defects, and valvular defects.
‡ Included G-tube, rectal atresia, and laryngomalacia.
† Included epilepsy, lissencephaly, microcephaly, hemiparesis, and myopathy.
§ Included Fragile X, Angelman, Pierre Robin, Timothy, Mowat–Wilson, and Robinow syndromes.
Table 2. Propensity-matched cohorts

Values are median (interquartile range) or n (%).
Table 3 summarises perioperative outcomes across both cohorts. The median hospital length of stay for the entire cohort was 1.22 days and differed according to surgical approach, with shorter duration in the thoracoscopy group compared to the thoracotomy group in both the unmatched (1.05 versus 2.41 days; p < 0.001) and propensity-matched cohorts (1.25 versus 2.27 days; p < 0.001). Quantile regression using inverse probability weighting by the propensity score demonstrated an estimated adjusted difference in median hospital length of stay of 1.26 days (p < 0.001). The median ICU length of stay for the entire cohort was 0 days and was also significantly shorter in the thoracoscopy group, unmatched (0 versus 0.75 days; p < 0.001) and matched (0 versus 0.86 days; p = 0.01, respectively). In addition, the odds of an ICU stay of at least 1 day was 4.09× greater in the thoracotomy group (95% confidence interval: 1.18–14.18; p = 0.026). The median operative time for the entire cohort was 69 minutes and was longer in patients undergoing thoracoscopy compared to thoracotomy (87 versus 56 minutes; p < 0.001). The same relationship held for the matched cohorts (81 versus 56 minutes; p = 0.007).
Table 3. Technical indices

Abbreviations: VATS = video-assisted thoracoscopic surgery; IQR = interquartile range.
* Ductal bleeding.
** Post-op arrhythmia.
In the entire cohort, seven patients required conversion from thoracoscopy to thoracotomy, six due to poor exposure and one due to ductal bleeding. Of the six cases involving poor exposure, one involved an atrio-pulmonary window-like patent ductus arteriosus, one required an extra-large clip (not available at that procedure) due to the size of the ductus, and the remainder were due to hyper-inflated lungs limiting visualisation. Six conversions were retained in the propensity-matched cohort.
Early post-operative outcomes were compared between patients undergoing thoracoscopy and thoracotomy. The residual rate was not significantly different between the two groups, although there was a trend towards higher residuals in the thoracoscopy group (6% thoracoscopy versus 0% thoracotomy; p = 0.344). In the propensity-matched cohorts, a similar trend was detected (6% thoracoscopy versus 0% thoracotomy; p = 0.571). Of the seven residuals in the thoracoscopy group that were detected prior to discharge, one was found during intra-operative transoesophageal echocardiography and required conversion, and two were discovered post-operatively and required re-operation. Following change in practice to use of the Yasargil clip for ligation, residual defects have not occurred. No significant differences were detected for other operative complications, including bleeding, pneumo- or chylothorax, and laryngeal nerve injury, save for isolated chest tube placement, which was more frequent in both the unmatched thoracotomy cohort (50% versus 11%; p < 0.001) and matched thoracotomy cohort (60% versus 17%; p < 0.001). Of note, no significant difference in post-operative outcome or morbidity was found in patients who required conversion compared to those who did not.
Follow-up outcomes are summarised in Table 4. For the entire cohort, the rate of follow-up was 58% and median follow-up time was 1 year. Although three new residuals were detected at follow-up, all in the thoracoscopy group (3.8%), the rate of new residuals did not differ significantly between the thoracotomy and thoracoscopy groups both before and after matching (p = 0.999). The same was true for the residual rate across all episodes of care – from post-operative to follow-up – although there was a trend towards more residuals following thoracoscopy (unmatched, 7.9% versus 0%; p = 0.064 and matched, 10.1% versus 0%; p = 0.342). In terms of other adverse outcomes, one patient in the thoracotomy group suffered permanent vocal cord injury and two patient deaths occurred that were unrelated to a patent ductus, one due to primary pulmonary hypertension in the thoracoscopy group, and one due to epilepsy in the thoracotomy group. There was no significant difference in freedom from re-intervention in the unmatched cohort (98% thoracoscopy versus 100% thoracotomy; p = 0.999) as well as the matched cohort (96% thoracoscopy versus 100% thoracotomy; p = 0.999.
Table 4. Outcomes at follow-up

All outcomes were assessed using only patients with documented follow-up.
Abbreviations: VATS = video-assisted thoracoscopic surgery.
* Mortality from primary pulmonary hypertension (VATS) and epilepsy (thoracotomy).
Discussion
Since Laborde and colleagues first described the use of video-assisted thoracoscopic surgery for persistent patent ductus arteriosus in 1993, the approach has gained appeal in the paediatric surgical community.Reference Laborde, Noirhomme, Karam, Batisse, Bourel and Saint Maurice10 However, there remains a dearth of literature comparing outcomes between thoracoscopy and thoracotomy, particularly in patients undergoing elective management of patent ductus arteriosus. This retrospective cohort study found that both approaches are safe and effective, with similarly low rates of complication and freedom from re-operation. Our data demonstrated that thoracoscopy was associated with longer operative times but resulted in significantly shorter hospitalisations and ICU stays, as well as fewer chest tube placements. Although patients undergoing thoracoscopy were significantly older than patients undergoing thoracotomy, the differences in length of stay persisted even after adjusting for potential baseline confounders with propensity score matching.
While this study found an association between thoracoscopy and longer operative times, other studies have demonstrated either no differenceReference Kennedy, Snyder, Ashcraft and Manning5,Reference Esfahanizadeh, Aghaee Meybodi and Sepehri Shamloo8,Reference Stankowski, Aboul-Hassan, Marczak, Szymanska, Augustyn and Cichon9 or shorter duration.Reference Vanamo, Berg, Kokki and Tikanoja6,Reference Chen, Weng and Chen7 This discrepancy can likely be attributed to the learning curve associated with the procedure, as most patent ductus arteriosus closures done at Boston Children’s Hospital are by thoracotomy, with premature infants representing the vast majority of cases. In contrast, other centres have cited case volumes numbering in the hundreds to thousands, with Nezefati et al most notably reporting a total of 2000 cases with a mean skin-to-skin time of 10 ± 2 min.Reference Nezafati, Soltani, Mottaghi, Horri and Nezafati4 In similar procedures using thoracoscopic techniques, it has been shown that surgeons need to perform at least 50 procedures to feel comfortable, with even higher case volumes needed to decrease operative times.Reference McKenna11,Reference Hsieh, Wen, Fang, Wen, Lin and Chao12 At Boston Children’s Hospital, the case volume per surgeon was not sufficient to trend operative times against experience, but with more clinical data, it would not be unreasonable to expect shorter operative times. Taken together, our data and that reported in the literature suggest that increased utilisation may eliminate differences in operative time compared to thoracotomy, while preserving the benefits of shorter hospitalisations and ICU stays.
The association between thoracoscopy and shorter hospitalisation has been observed in prior studiesReference Vanamo, Berg, Kokki and Tikanoja6,Reference Esfahanizadeh, Aghaee Meybodi and Sepehri Shamloo8 as well as other lesions, including vascular rings.Reference Herrin, Zurakowski, Fynn-Thompson, Baird, Del Nido and Emani13 However, there is some disagreement in the literature, as other groups have reported no difference, although these either had fewer patient numbers or had focused on neonates and infants rather than patients receiving elective surgery.Reference Kennedy, Snyder, Ashcraft and Manning5,Reference Chen, Weng and Chen7 In contrast, there does appear to be general agreement regarding an association between thoracoscopy and reduced ICU stay.Reference Vanamo, Berg, Kokki and Tikanoja6,Reference Esfahanizadeh, Aghaee Meybodi and Sepehri Shamloo8,Reference Stankowski, Aboul-Hassan, Marczak, Szymanska, Augustyn and Cichon9 One contributing factor may be the significantly lower rate of chest tube placement observed in this study, which has been observed previouslyReference Stankowski, Aboul-Hassan, Marczak, Szymanska, Augustyn and Cichon9; other factors may include reduced post-operative pain and blood loss compared to thoracotomy.Reference Miles, DeLeon and Muraskas14,Reference Bolourian15 Along with implications for patient satisfaction, the faster recovery times associated with thoracoscopy may also have cost benefits, as reported by Chen et al.Reference Chen, Weng and Chen7 Aside from chest tube placement, we observed no other significant differences in complication rates in both the unmatched and matched cohorts, including residuals, vocal cord injury, pneumothoraxes, and rate of re-intervention.
However, while not statistically significant, we did observe a trend towards higher residuals in the thoracoscopy group before and after matching. Although most residuals were not re-intervened upon, and freedom from re-operation was similar to prior studies,Reference Jacobs, Giroud and Quintessenza16 we still recommend confirmatory intraoperative transoesophageal echocardiography for all closures, as adoption of this approach has decreased our rate of residuals (three detected post-operatively in the last 10 years). While the 6% conversion rate observed here fell within the range reported in the literature (0.75% to 14.6%) and did not lead to higher rates of complications, it remains a non-negligible risk in the thoracoscopic approach – as do residuals – and one of its potential downsides.Reference Nezafati, Soltani, Mottaghi, Horri and Nezafati4,Reference Jacobs, Giroud and Quintessenza16 Reasons for conversion included poor exposure due to ductus size or shape, lung hyperinflation, and ductal bleeding and were similar to prior studies.Reference Esfahanizadeh, Aghaee Meybodi and Sepehri Shamloo8,Reference Stankowski, Aboul-Hassan, Marczak, Szymanska, Augustyn and Cichon9 Thus, for surgeons with less experience, it may be prudent initially to choose the thoracoscopic approach primarily in lower risk patients and to ensure appropriate lung ventilation during the procedure. With increasing procedural experience, the risk of conversion or residual is likely to decrease, though this would need to be verified in a future study. At our institution, we have also transitioned to using the Yasargil clip (Fig 1), which provides extended reach in cases where a traditional clip may not deploy completely around a ductus and offers calibrated closing forces not seen with traditional clips, reducing the risk of ductal fracture and bleeding. Furthermore, the Yasargil clip is less abrasive at its terminus, potentially decreasing the risk of recurrent laryngeal nerve injury, which has been observed at rates as high as 6%.Reference Vanamo, Berg, Kokki and Tikanoja6
With regard to increasing utilisation and case volumes, it should be noted that video-assisted thoracoscopic instruments for the paediatric population have not been standardised or well developed. Specifically, the current technology encourages custom-made and adult-oriented instruments that are not easily translatable to the paediatric context, and this may be one of the reasons why the technique has not been more universally adapted. Further development in paediatric-specific thoracoscopic tools may improve rates of adoption among the surgical community and may lead to decreased cost, as mentioned above. Concomitantly, more widespread utilisation of video-assisted thoracoscopic surgery may accelerate instrument standardisation.
Another alternative to thoracotomy that has seen increasing utilisation is transcatheter closure of patent ductus arteriosus. Although this method has become standard of care in many institutions, video-assisted thoracoscopic surgery may be a viable alternative when transcatheter device closure is not feasible. In our institution, the decision to close via catheterisation or surgical closure is based on ductal anatomy – if short and broad, we elect to proceed via the latter, based on American Heart Association (AHA) recommendations and investigations which have demonstrated worse outcomes in large ductuses.Reference Feltes, Bacha and Beekman17,Reference Fu, Hwang and Jan18 In our study, we observed that nine patients (six thoracoscopy and three thoracotomy) had previously failed attempts at transcatheter device closure, some due to large ductal size and others due to aortopulmonary window-like anatomy. A head-to-head comparison of thoracoscopy and catheter occlusion was outside the scope of this investigation, but in light of ongoing debate regarding outcomes such as efficacy, complication rates, and resource utilisation, future work should seek to undertake this direct comparison. For example, while a recent meta-analysis has shown that re-intervention is more common in catheter-based treatment than surgical ligation, length of stay may be longer for surgical ligation.Reference Lam, Lopushinsky, Ma, Dicke and Brindle19,Reference Chen, Weng and Chen20
This study had a number of limitations. First, it was conducted at a single institution, which may be associated with institutional bias. Although it is unlikely that operative approaches for patent ductus arteriosus closure differ widely across institutions, it is important to note that the current literature is comprised of single-centre studies that were performed in disparate countries and that the instruments and techniques utilised vary. To this end, this study provides the largest United States-based comparison between thoracoscopy and thoracotomy to date. Future studies would benefit from a detailed examination of the various approaches that are undertaken among the various countries and centres from which the literature is derived. Similarly, an international registry to enable multilateral follow-up and to standardise treatment approaches would be helpful, with the additional benefit of propelling advancement in paediatric thoracoscopy instrumentation. Second, although the patient cohorts in this study achieved adequate power, we recommend that a multi-centre prospective study for thoracoscopic intervention be undertaken to confirm the results of this study. We also acknowledge that the original cohorts prior to propensity matching had different baseline characteristics. While we did adjust for these differences using propensity matching and observed concordance in our findings between the unmatched cohorts and the matched cohorts to support our conclusions, it is possible that there were other factors not adjusted for that could have confounded our results. However, if this were the case, we do not believe that there was a marked effect, given that our results are generally in agreement with prior studies. Finally, it is possible that variability in surgeon experience may have influenced the results, and future studies may seek to elucidate the effect of the learning curve on operative outcomes for patent ductus arteriosus closure.
In conclusion, video-assisted thoracoscopic patent ductus arteriosus ligation offers a safe and effective alternative to thoracotomy. Although shorter operative times were observed in the thoracotomy cohort, this may have been due to a learning curve rather than the nature of thoracoscopy itself. Potential downsides may include conversions and higher residual rates, and surgeons still accruing experience may benefit from selection of more low-risk patients. Regardless of surgeon experience however, intraoperative monitoring is crucial, including transoesophageal echocardiography. Additional benefits of the thoracoscopic approach include decreased incidence of chest tube placement, shorter hospital stay, and reduced ICU stay.
Acknowledgements
Authors as listed.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
Not applicable.