Patients with single ventricles are typically managed through multi-stage palliation, with an intermediate stage of bidirectional cavopulmonary connection, and concluding with the total cavopulmonary connection or Fontan circulation. At each stage of palliation, haemodynamically significant lesions may develop as a consequence of patient growth, or occur as residua of previous surgeries, and therefore compromise the outcome of subsequent surgeries.
The common potential lesions requiring amelioration around the time of bidirectional cavopulmonary connection include the following: atrioventricular valve regurgitation, recoarctation of the aorta, branch pulmonary artery stenosis, or restrictive intra-atrial communication. The identification of such problems may prompt treatment in advance or at the time of surgery. Typical imaging modalities used for surveillance before bidirectional cavopulmonary connection include diagnostic cardiac catheterisation, echocardiography, cardiac MRI, and CT.
Previous studies have raised concerns about the ability of echocardiography to visualise vascular stenoses in this population: Stern et alReference Stern, McElhinney, Gauvreau, Geva and Brown 1 demonstrated the superiority of cardiac catheterisation over an echocardiography-only imaging strategy. A randomised controlled trial of diagnostic catheterisation compared with MRI established the safety and efficacy of MRI for the imaging of patients before bidirectional cavopulmonary connection, at lower cost and with fewer minor complications.Reference Brown, Gauvreau and Powell 2 , Reference Brown, Gauvreau and Powell 3
Despite good evidence for the efficacy of an MRI-based imaging strategy for pre-bidirectional cavopulmonary connection assessment, it is possible that echocardiography could provide additional and independent data about certain lesions, when used in combination with MRI. In our centre, a composite imaging strategy using MRI and echocardiography is used by default, with CT scanning and cardiac catheterisation reserved for patients requiring urgent, early imaging or intervention, respectively.
In this study of patients undergoing bidirectional cavopulmonary connection, we sought to determine the relative contributions of MRI and echocardiography as part of a composite imaging strategy for the diagnosis of lesions requiring intervention.
Methods
Between June, 2007 and December, 2012, 109 patients underwent bidirectional cavopulmonary connection at Great Ormond Street Hospital for single-ventricle palliation. Of these, 72 consecutive patients, who underwent pre-operative assessment using both echocardiography and MRI, were included in this study. The remaining patients were assessed by echocardiography; If based on echocardiography the lesion was deemed highly likely to require early percutaneous intervention, patients underwent cardiac catheterisation without preceding MRI investigation. If additional data regarding airway adequacy or pulmonary pathology was required, then CT scanning was performed rather than MRI. Having acquired comprehensive interventional catheterisation or CT data, many patients did not require additional MRI imaging before bidirectional cavopulmonary connection surgery (Fig 1).
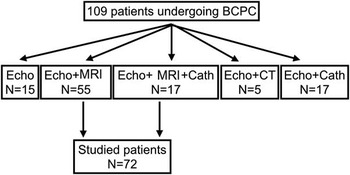
Figure 1 The imaging modalities used in all patients during pre-operative assessment for bidirectional cavopulmonary connection. Cath=cardiac catheterisation; Echo=echocardiography.
Informed consent for the use of imaging data was obtained from all parents/guardians of the patients included in this study. The study was retrospective; however, imaging data from clinical imaging studies were re-analysed for the purposes of this study.
Outcome measures
Significant lesions were defined as those requiring specific surgical or catheter intervention in the peri-bidirectional cavopulmonary connection period. This time frame included interventions performed at the time of bidirectional cavopulmonary connection, or as rescue of untreated lesions within the 6 months following bidirectional cavopulmonary connection.
In our unit, the decision for any intervention was made within the forum of a structured, multidisciplinary team discussion. Consensus agreement to intervene was required, and the decisions were based on the cumulative evidence from all available clinical, haemodynamic, and imaging data at the time.
Lesions requiring intervention were categorised into the following groups: left or right branch pulmonary artery stenosis, aortic arch recoarctation, restriction of the interatrial septum, and significant atrioventricular valve regurgitation. The occurrence of an intervention on a given structure was recorded as a binary outcome. The relationship between this outcome and the echocardiographic or MRI imaging assessment of the structure was assessed by binary logistic regression analysis.
Echocardiography technique
Echocardiography was performed before bidirectional cavopulmonary connection surgery using either Vivid 7 (GE Medical Systems, Milwaukee, Wisconsin, United States of America) or Philips i33 (Philips, Amsterdam, The Netherlands) scanners with multi-frequency 3–10 MHz probes.
Out of 72 patients, 46 (64%) were scanned under the same general anaesthetics as for MRI, and the remaining 26 (36%) patients underwent echocardiography scans without anaesthetics within a few days of MRI scanning. The scans were comprehensive, morphological, and functional assessments according to internal segmental sequentional protocol. All subcostal, parasternal, and suprasternal projections were used to achieve the best possible imaging quality. Images of the pulmonary branches were obtained from the suprasternal approach, of the right pulmonary artery in the standard horizontal plane (“3 o’clock”), and the left pulmonary artery was visualised in the slightly posterior and left angulation (“posterior 2 o’clock”).
In cases where patients underwent balloon angioplasty of recoarctation of the aorta before bidirectional cavopulmonary connection, the examination performed before this intervention was analysed.
MRI technique
MRI was performed under general anaesthesia as previously describedReference Muthurangu, Taylor and Hegde 4 – Reference Stockton, Hughes, Broadhead, Taylor and McEwan 6 using a 1.5T MRI scanner (Avanto, Siemens Medical Systems, Erlangen, Germany). A three-dimensional balanced steady-state free precession sequence was used to assess intra-cardiac anatomy. This involved three-dimensional MRI images covering the entire heart, obtained during diastole in a sagittal orientation, using a magnetisation-prepared three-dimensional balanced steady-state free precession sequence with navigator respiratory gating. Great artery vessel anatomy was also assessed using a gadolinium-enhanced MRI angiography three-dimensional fast-field-echo sequence. Gadolinium (Dotarem; Guerbet LLC, Bloomington, Indiana, United States of America) was injected into a peripheral vein and tracked into the heart with a dynamic coronal two-dimensional fast-field-echo sequence. The gadolinium dose was 0.4 mmol/kg. The MRI angiographic sequence was initiated when the contrast reached the ventricle; two consecutive angiograms were acquired in a single 15- to 20-second period of apnoea.
MRI ventricular function
Retrospectively gated, steady-state free precession cine MRI of the heart was acquired using breath-holding – in the vertical long-axis, four-chamber, and the short-axis planes – covering the entirety of both ventricles (9–12 slices). Assessment of single-ventricle volumes was performed by manual segmentation of short-axis cine images at end diastole and end systole (OsiriX; OsiriX Foundation, Geneva, Switzerland). End-diastolic and end-systolic volumes were calculated using Simpson’s rule, and from these volumes stroke volume was calculated.
MRI quantification of flow and atrioventricular valve regurgitation
Through-plane flow data were acquired in the superior caval vein, inferior caval vein, pulmonary trunk, when present, branch pulmonary arteries, pulmonary veins, and ascending aorta, using a flow-sensitive gradient-echo sequence during (ventilated) free breathing and with retrospective cardiac gating. Arterial and venous blood flow was calculated from phase contrast images using a semi-automatic vessel edge-detection algorithm (OsiriX) with operator correction. All volume and flow measurements were indexed for body surface area. The atrioventricular valve regurgitation fraction was calculated as the difference between the stroke volume of the single ventricle and forward flow of the systemic artery divided by the stroke volume of the single ventricle.
Measurements performed using echocardiography and MRI scans
Measurement of pulmonary arteries
Pulmonary artery branches were measured at two sites as follows: proximal, measured close to the pulmonary artery bifurcation; and distal, measured before the second-order branches. In case of stenosis, the smallest dimension was used. For echocardiography, these measurements were made offline, using calipers, from two-dimensional images of the longitudinal axis of the vessel. For MRI, these measurements were made using OsiriX 3D post-processing software in order to produce a multi-planar reconstruction giving the en-face vessel outline. Vessel diameters were measured across this true cross-sectional short axis, with the software calipers (Fig 2). In the absence of robust local or published normal data from MRI for infants, both the echocardiography and MRI measurements were converted to a Z score using the Detroit dataReference Pettersen, Du, Skeens and Humes 7 for echocardiographic measurements.
Branch pulmonary arteries were classified as hypoplastic if the Z score of both proximal and distal parts was <−2. The stenosis of the branches was classified as mild if the narrowest segment was 20–40% smaller than the adjacent segment, as moderate if 40–60%, and as severe if there was a 60% difference between both parts of the pulmonary branch.Reference Muthurangu, Taylor and Hegde 4 In cases where image quality prevented assessment, data were scored as no stenosis (Score 0). The following scoring system was used: no stenosis of the pulmonary arteries=0, mild stenosis=1, moderate stenosis=2, severe stenosis=3, and hypoplasia of the pulmonary arteries=4.
Where image quality did not allow accurate measurement of the branch pulmonary artery, it was encoded as “no stenosis”, for the purposes of statistical analysis using binary logistic regression. In addition to the geometric classification, a second MRI parameter was used – “MRI conclusion” – which was based on experienced MRI reporters’ overall assessment of the branch pulmonary arteries. In addition to the dimensions of the vessel, additional data considered included asymmetric distribution of flow volume on branch pulmonary arteries or bilateral pulmonary venous flow measurements or flow turbulence visualised on cine imaging of the branch pulmonary arteries. The MRI conclusions were based on standardised unit criteria, and were formed by the four experienced MRI consultants, each with at least 7 years of experience.
Measurement of aorta
For the purpose of this study, the aortic isthmus – just distal to the left subclavian artery – and abdominal aorta – at the level of the diaphragm – were measured by echocardiography and MRI (Fig 3). The coarctation index was calculated from the ratio of the dimensions of the aortic isthmus and abdominal aorta. Mild coarctation was classified if the coarctation index was 0.51–0.75, moderate if the index was 0.26–0.50, and severe when the index measured <0.25.Reference Hughes, Tsang and Kostolny 8 , Reference Lemler, Zellers, Harris and Ramaciotti 9
Where image quality did not allow accurate measurement of the aorta, it was encoded as “no stenosis”, for the purposes of statistical analysis using binary logistic regression.
As for the branch pulmonary arteries, experienced MRI reporters recorded a second MRI parameter, “MRI conclusion”, based on the overall assessment of the aortic arch.
Evaluation of the interatrial communication
The haemodynamic significance of restriction of the interatrial communication was qualitatively assessed by echocardiography, using the two-dimensional and colour Doppler loops, and by MRI using cine imaging. The interatrial communication was classified as unrestrictive, moderately restrictive, or severely restrictive. This was based on the reporter’s overall conclusion, using the cumulative parameters of septal deviation, defect size, shunt flow turbulence, and velocity.
Evaluation of atrioventricular valve regurgitation
Atrioventricular valve regurgitation was assessed by echocardiography from the colour Doppler loops. Atrioventricular valve regurgitation was divided into four groups: 0–3, where grade 0 was used if no regurgitation was present on the echocardiographic images and grade 3 for severe regurgitation. MRI used the calculated regurgitation fraction for the assessment of the atrioventricular valve regurgitation. Regurgitation fraction between 10 and 20% was classified as mild, between 20 and 40% as moderate, and >40% as severe.
Statistics
Using the requirement for an intervention as a binary outcome, and the echocardiographic or MRI assessments as explanatory variables, binary logistic regression analysis was carried out to estimate the predictive value of each modality. A 95% confidence interval was considered, and a p value of <0.05 was assumed significant. Stepwise multivariable binary logistic regression was used to assess the independent association of combinations of explanatory variables with the binary outcome. The likelihood ratio test was performed to gauge the contribution of individual predictors to the resulting models, and the Nagelkerke’s R2 was calculated to assess the appropriateness of the models’ fit. In this way, the covariates associated with the risk of intervention were identified. The analysis was carried out using the commercial software IBM SPSS Statistics (IBM Corp., New York, United States of America).
Results
Patient demographics
Out of 109 patients undergoing bidirectional cavopulmonary connection between 2007 and 2012, a total of 72 patients underwent both echocardiography and MRI pre-operatively, and therefore fitted the recruitment criteria. Of these 72, the median age at the time of bidirectional cavopulmonary connection was 160 days (interquartile range 121–284), and the median weight was 5.4 kg (interquartile range 4.9–6.9).
The systemic ventricle had left ventricular morphology in 33 patients (46%) and right ventricular morphology in 39 patients (54%). Clinical diagnoses included the following: hypoplastic left heart syndrome (28 patients), tricuspid atresia (seven patients), double-inlet ventricle (nine patients), pulmonary atresia with intact ventricular septum (seven patients), criss-cross heart (three patients), unbalanced atrioventricular septal defect (two patients), corrected transposition of the great arteries (two patients), right atrial isomerism (two patients), double-outlet right ventricle (two patients), Shone syndrome (two patients), critical pulmonary stenosis (two patients), and other complex CHD in another six patients.
All but two patients had undergone a previous first-stage surgical palliation; 22 patients had required at least one additional intervention before bidirectional cavopulmonary connection.
Altogether, four patients died during the follow-up period. The deaths occurred at the age of 2 months, 3 months, 10 months, and 5 years, respectively. None of the deaths was related to MRI/echocardiography misdiagnosis; three patients suffered from significant co-morbidity. The first patient suffered from multi-organ failure, with multiple pulmonary cysts. The second patient had septic shock after mechanical atrioventricular valve replacement. The third patient had severe right ventricular dysfunction and developed cardiogenic shock after fundoplication. The last patient died after takedown of Fontan circulation and subsequent circulatory arrest and brain insult.
Table 1 shows the concomitant procedures relevant to the measured echocardiography and MRI parameters performed in the peri-bidirectional cavopulmonary connection period. In total, 10 patients required at least one further intervention during the 6 months after bidirectional cavopulmonary connection.
Table 1 Procedures performed before or at the time of bidirectional cavopulmonary connection (BCPC) and during the first 6 months after BCPC.
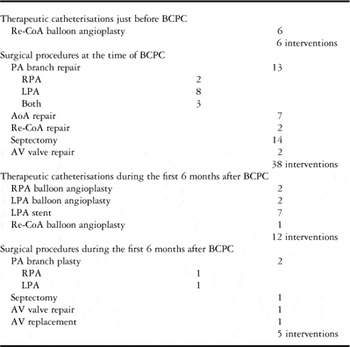
AoA=aortic arch; AV=atrioventricular; LPA=left pulmonary artery; PA=pulmonary artery; Re-CoA=recoarctation of the aorta; RPA=right pulmonary artery
Right pulmonary artery intervention
In total, eight right pulmonary artery interventions were performed in eight patients: five surgical augmentations of the right pulmonary artery at the time of bidirectional cavopulmonary connection, two balloon angioplasties, and one surgical augmentation within 6 months after bidirectional cavopulmonary connection.
The right pulmonary artery could not be measured by echocardiography in 13 patients (18%) because of poor image quality.
In univariable and multivariable analyses (Table 2), the MRI right pulmonary artery Z score only (odds ratio 1.77, 95% confidence interval 1.12–2.79) was associated with the requirement for intervention. Echocardiographic assessment was not associated with right pulmonary artery intervention. A logistic regression model based on MRI right pulmonary artery Z score was found to correctly predict intervention in 85% of patients using a predicted probability score of 0.3.
Table 2 Binary logistic regression analysis of intervention on the right and left pulmonary arteries, aortic arch, interatrial septum (IAS), and atrioventricular (AV) valve.
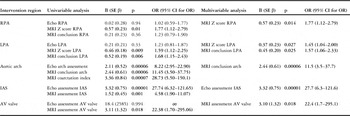
CI=confidence interval; Echo=echocardiography; LPA=left pulmonary artery; OR=odds ratio; RPA=right pulmonary artery
The left-hand columns show the individual association of imaging covariates with the binary outcome; significant variables are shown in bold. The right-hand columns show the results of the stepwise multivariable binary logistic regression; covariates independently and significantly associated with the outcome are shown
Left pulmonary artery intervention
In total, 21 left pulmonary artery interventions were performed in 14 patients. At the time of bidirectional cavopulmonary connection, 11 patients underwent left pulmonary artery surgical augmentation, 3/11 required further balloon angioplasty and stenting during the 6-month follow-up period, and 2/11 required two catheter re-interventions including stenting. Moreover, three patients required left pulmonary artery intervention after bidirectional cavopulmonary connection – two stent placements and one surgical augmentation.
The left pulmonary artery dimensions could not be measured by echocardiography in 14 patients (19%) because of inadequate image quality.
MRI left pulmonary artery Z score (odds ratio 1.59, 95% confidence interval 1.12–2.25) and MRI conclusion of left pulmonary artery stenosis (odds ratio 1.68, 95% confidence interval 1.15–2.43) were associated with correct prediction of left pulmonary artery intervention by univariable analysis (Table 2). The echocardiographic left pulmonary artery Z score was not significantly associated with intervention.
A multivariable regression model (Table 2) was fitted to the data using the two MRI covariates – Z score and conclusion. These were found to be independently associated with the need for intervention. Using this model, a predicted probability score of 0.50 correctly predicted intervention or no intervention in 80% of the patients.
Aortic arch examination versus intervention
In total, 15 patients required aortic arch intervention: 10 with hypoplastic left heart syndrome, three with double-inlet ventricle, one with Shone syndrome, and one with tricuspid atresia. Balloon dilation of the recoarctation was performed in six patients at pre-bidirectional cavopulmonary connection catheterisation. Surgical relief of recoarctation as the only aortic arch procedure (two patients) or as a part of complex aortic arch surgery (seven patients) was performed in another nine patients. Another patient required balloon dilation of recoarctation during the 6-month follow-up period; one patient received two aortic arch procedures, balloon dilation followed by surgery.
Inadequate image quality did not allow assessment of aortic arch dimensions by echocardiography in 12 patients (17%).
MRI coarctation index (odds ratio 28.73, 95% confidence interval 5.50–150.1), MRI conclusion (odds ratio 11.45, 95% confidence interval 3.50–37.75), and arch assessment by echocardiography (odds ratio 8.22, 95% confidence interval 2.92–22.90) were each associated with the need for arch intervention determined by univariable analysis (Table 2).
Multivariable logistic regression analysis (Table 2) identified that MRI arch conclusion only was independently associated with arch intervention (odds ratio 11.5, 95% confidence interval 3.5–37.7). Using this model, a predicted probability score of 0.50 correctly predicted intervention or lack of it in 90% of the patients.
Interatrial septum examination versus intervention
Among all, 14 patients required atrial septectomy at the time of bidirectional cavopulmonary connection. In one patient, repeated septectomy was performed during the 6-month follow-up period.
Univariable analysis (Table 2) showed an association between the echocardiographic assessment and need for septectomy (odds ratio 27.74, 95% confidence interval 6.32–121.65) and between the MRI assessment and need for septectomy (odds ratio 4.58, 95% confidence interval 1.90–11.07).
A multivariable analysis (Table 2) demonstrated that only echocardiographic assessment of the interatrial communication predicted septectomy (odds ratio 27.7, 95% confidence interval 6.3–121.6). Using this model, a predicted probability score of 0.50 correctly predicted intervention or no intervention in 96% of the patients.
Examination of atrioventricular valve competence versus intervention
Among all, three patients required atrioventricular valve intervention – two patients at the time of the bidirectional cavopulmonary connection and one patient during the 6-month follow-up period; one patient required atrioventricular valve replacement within 6 months after an initial atrioventricular valve intervention performed at the time of bidirectional cavopulmonary connection.
Univariable analysis (Table 2) showed an association between the need for atrioventricular valve intervention and MRI regurgitation fraction (odds ratio 22.38, 95% confidence interval 1.70–295.1). There was no association with echocardiographic assessment of atrioventricular valve regurgitation.
A multivariable analysis (Table 2) demonstrated that only MRI regurgitation score predicted intervention (odds ratio 22.4, 95% confidence interval 1.7–295.1). Using this model, a predicted probability score of 0.50 correctly predicted intervention or no intervention in 97% of patients.
Limitations
The main limitation of this study is that the outcome measure was a surgical or catheter-based intervention on a pre-defined group of lesions. Therefore, this involves a patient-specific and centre-specific threshold for intervention, which may not be standardised or represent practice in different centres – for example, an intervention performed is not the gold standard criteria for definition of a haemodynamically significant lesion. Therefore, analysis of this study cannot be considered simply in terms of sensitivity and specificity of the diagnostic imaging.
This study did not address any differences between MRI and catheter findings, because confounding was highly likely. In our unit, cardiac catheterisation was carried out specifically when there was a high probability for intervention, thus only a limited and clinically skewed proportion of the cases underwent both investigations. In addition, the temporal relationship between cardiac catheterisation and MRI was not always consistent, and blinding of the clinical reporters from the results of each investigation was not possible.
Discussion
In this era of rapidly advancing techniques for cardiac imaging and multi-modality assessment, this study successfully examined the current relative contribution of two imaging modalities to the diagnosis of lesions requiring intervention within the peri-bidirectional cavopulmonary connection period. Our data indicate that, for the branch pulmonary arteries, the aortic arch, and atrioventricular valve regurgitation, the MRI assessment was superior to echocardiography for predicting intervention; however, for intervention on the interatrial septum, echocardiography was superior to MRI.
There are several known risk factors for failure of the bidirectional cavopulmonary connection in patients with single-ventricle physiology. These include surgery at a very young age (<3 months), dominant right ventricle, elevated pulmonary arterial pressure, and ventricular dysfunction.Reference Alsoufi, Manlhiot and Al-Ahmadi 10 – Reference Kogon, Plattner, Leong, Simsic, Kirshbom and Kanter 14
In addition, there are haemodynamic lesions that increase risk of mortality and morbidity of the bidirectional cavopulmonary connection, but can be successfully treated before or at the time of bidirectional cavopulmonary connection. These include branch pulmonary artery stenosis, recoarctation, atrioventricular valve regurgitation, and restriction of the interatrial septum.
Recoarctation is associated with worse right ventricular systolic function at the time of second-stage operation and more significant atrioventricular valve regurgitation in patients with hypoplastic left heart syndrome, but intervention on the recoarctation results in improvement of right ventricular function.Reference Januszewska, Kozlik-Feldmann and Kordon 15 , Reference Larrazabal, Selamet Tierney and Brown 16 Atrioventricular valve regurgitation is an important risk factor for patients with hypoplastic left heart syndrome.Reference Lee, Aiyagari, Hirsch, Ohye, Bove and Devaney 17 , Reference Carlo, Carberry and Heinle 18 Successful atrioventricular valve plasty improves the outcome in patients with single ventricle,Reference Honjo, Atlin and Mertens 19 possibly because of the high incidence of structural anomalies of the atrioventricular valveReference Honjo, Mertens and Van Arsdell 20 in this population. A non-restrictive interatrial communication is one of the important factors for maintaining adequate cardiac output in patients with single ventricle. Patients with hypoplastic left heart syndrome have higher mortality with restrictive interatrial communication compared with the non-restrictive type.Reference Photiadis, Urban and Sinzobahamvya 21
It is therefore crucial to diagnose these lesions in the most safe and effective way.
This study is in keeping with previous studies that have cautioned against reliance on echocardiography for the identification of vascular stenosis in single-ventricle patients;Reference Stern, McElhinney, Gauvreau, Geva and Brown 1 , Reference Muthurangu, Taylor and Hegde 4 one of the advantages of MRI is the ability to assess the dimensions of the vessels in a truly cross-sectional plane, perpendicular to the vessel, whereas echocardiography is limited to dimensions taken from suprasternal views only, gaining a longitudinal view of the vessel, in a craniocaudal (coronal) plane orientation. Thus, the dimension of the vessel measured in this single plane will be misleading with anything other than circular shape of the vessel.
Calculating the regurgitation fraction using ventricular stroke volumes and the stroke volume of systemic artery flow allows accurate quantification of the atrioventricular regurgitation with MRI. Conversely, echocardiography uses a more subjective, semi-quantitative assessment of the atrioventricular valve regurgitation, which depends on the instantaneous loading conditions, setting of the ultrasound machine, and experience of the examiner.
Nevertheless, our data provide support for the advantage of a composite imaging strategy. Although MRI appears more specific than echocardiography for the assessment of vascular stenoses and atrioventricular valve regurgitation, echocardiography provides a more reliable assessment of the adequacy of the interatrial communication. This may be related to differences in the specific interrogation of the interatrial septum in both imaging modalities. Echocardiography colour Doppler examination may be more sensitive to subtle changes in flow kinetics within low-pressure chambers. MRI cine imaging, often used to visualise the atrial septum, relies on sequences designed to minimise signal artefacts from flow turbulence.
Our study did not investigate the contribution of diagnostic catheterisation in this patient group. Recent data have shown that the short- and medium-term outcomes of patients investigated by MRI are not different to those undergoing diagnostic catheterisation, but that MRI has lower costs and fewer minor complications.Reference Brown, Gauvreau and Powell 2 , Reference Brown, Gauvreau and Powell 3 Several studies showed no advantage of diagnostic catheterisation, in terms of patient clinical status in the peri-operative course,Reference Jones, Ditchfield and Cahoon 22 early post-operative period,Reference Brown, Gauvreau and Powell 2 and in long-term follow-up after bidirectional cavopulmonary connectionReference Brown, Gauvreau and Powell 3 when comparing patients examined by catheterisation and MRI. In addition, there is higher risk of peri-procedural complications in patients undergoing catheterisation.Reference Brown, Gauvreau and Powell 2 , Reference Brown, Gauvreau and Powell 3 For these reasons, in our cardiac centre, cardiac catheterisation is reserved for patients with a high likelihood of requiring intervention.
The outcome measure for this study was a surgical or catheter intervention on a pre-defined group of lesions. Crucially, this incorporates a patient-specific and centre-specific threshold for intervention, which may not be standardised, and may not represent practice in different centres. In our unit, the decision for any intervention was made within the forum of a structured, multidisciplinary team discussion. Consensus agreement to intervene was required, and the decisions were based on the cumulative evidence from all available clinical, haemodynamic, and imaging data at the time.
Any intervention performed is not gold standard criteria for definition of a haemodynamically significant lesion. Likewise, when a specific intervention is not performed, the clinical decision making not to intervene may have been influenced by factors other than the absolute haemodynamic significance of that single lesion. Therefore, this study cannot be considered or analysed in terms of the sensitivity and specificity of the diagnostic investigations.
This study demonstrates that for branch pulmonary artery stenosis, aortic arch coarctation, and atrioventricular valve regurgitation, MRI parameters more reliably predict the need for intervention; however, the adequacy of interatrial communication is more accurately identified by echocardiography. The complete assessment of patients with single-ventricle physiology approaching bidirectional cavopulmonary connection requires the cumulative strengths of multi-modality imaging. The relative strengths of MRI and echocardiography should be acknowledged when considering intervention.
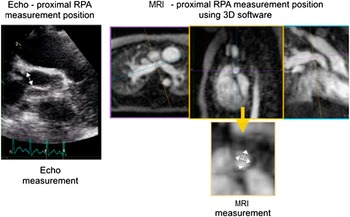
Figure 2 An illustration of the measurement technique of the proximal right pulmonary artery (RPA), for both echocardiography (Echo) and MRI. The white arrows show the dimensions recorded. The two cross-sectional measurements made from MRI data were averaged to achieve a single measurement, comparable with Echo.
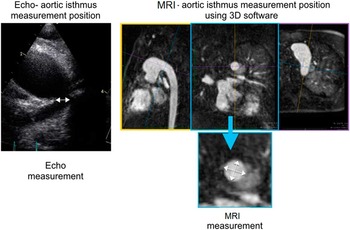
Figure 3 An illustration of the measurement technique of the aortic isthmus, for both echocardiography (Echo) and MRI. The white arrows show the dimensions recorded. The two cross-sectional measurements made from MRI data were averaged to achieve a single measurement, comparable with Echo.
Acknowledgements
This research study was supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for profit sectors.
Conflicts of Interest
None.
Ethical Standards
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institutional and research ethical committee (Great Ormond Street Hospital Research ethical code – 06/Q0508/124).








