Published online by Cambridge University Press: 24 May 2005
The purpose of my review is to present the current knowledge of the impact of the systemic inflammatory response on morbidity following cardiac surgery in children. In the first section, I describe the general principles of the systemic inflammatory response; in the second I consider the impact of cardiac surgery on the systemic inflammatory response. In the third section, I present examples illustrating the relevance of the systemic inflammatory response for postoperative morbidity in children. In the fourth section, I review therapeutic strategies to reduce the inflammatory response to cardiac surgery, and hence postoperative morbidity. Finally, in the last section, I discuss the influence of the preoperative condition on the systemic inflammatory reaction to cardiac surgery.
Systemic inflammation is the non-specific physiological response to a large variety of stimuluses. The systemic inflammatory response caused by bacterial or viral infections is the classical example for this. Systemic inflammation, however, is also elicited by non-infectious stimuluses. Ischemia and re-perfusion, trauma, endotoxaemia, and virtually all situations allowing contact between blood components of an individual and foreign surfaces are inflammatory stimuluses.
The blood, the liver and the brain are probably the most important organs controlling initiation and termination of the systemic inflammatory reaction.1, 2
The complement system is involved early in the complex process of inflammation. Like the systems underscoring coagulation, contact with plasma, and fibrinolysis, with which it has numerous interactions, it is activated in the fashion of a cascade.
The complement system may be activated by one of two pathways, the classical and the alternative, or by both. The classical pathway is mainly activated by antigen-antibody complexes, whereas the alternative pathway is activated by polysaccharides and polymers, such as those found in the cell membrane of microorganisms or those utilised for the fabrication of medical artificial surfaces. The activation of either pathway leads to the formation of C3-convertase, which activates C3, a key protein of the terminal sequence of the complement system. The split products of this activation are C3a and C3b. C3a is a potent anaphylatoxin. C3b is part of the C3-convertase of the alternative pathway, and of C5-convertase, which is common to both pathways. C5-convertase activates C5, the split products of which are C5a and C5b. C5a is the most potent anaphylatoxin. C5b, by binding the complement proteins C6 to C9 forms the terminal complex, also called the membrane attack complex.3 The process of activation of complement through either pathway, leading to the formation of the membrane attack complex, is shown in Figure 1.

Figure 1. The complement cascade can be activated throughout either the classical or the alternative pathways. The activation of the classical pathway begins with the binding of an activator to C1, which is composed of 3 portions C1q, C1r and C1s. C1 activates in turn C2 and C4, which are split into C2a, C2b and C4a, C4b, respectively. The split products C2a and C4b bind to form the C3-convertase of the classical pathway.
Figure 1. The activation of the alternative pathway probably depends upon cleavage of small amounts of C3 in C3a and C3b. C3b is then available for binding factor B. Factor D cleaves bound factor B to produce Ba and Bb. The complex C3bBb is the C3-convertase of the alternative pathway that is stabilised by factor P, also called properdine.
Figure 1. Both C3-convertases cleave C3 into C3a and C3b. By binding an additional C3b, both C3-convertases become C5-convertases. The cleavage of C5 produces C5a and C5b. C5b bounds C6 and in a stepwise fashion, C7, C8 and C9 are added into the complex to form the so called membrane attack complex. The main molecules with biological properties are represented in red (anaphylatoxins) and in blue (membrane attack complex).
The biologically active peptides released into the circulation (Table 1) increase vascular permeability, cause vasoconstriction, and lead to formation of oedema. In addition, they activate leukocytes, stimulate phagocytosis, and cause cytolysis.
Table 1. Biological properties of the main components of the complement system.

Leukocytes, which are major targets for activated complement proteins, contribute to sustain systemic inflammation. Upon stimulation, they release products of degranulation such as elastase, myeloperoxydase and histamine, and synthesise new inflammatory mediators, such as cytokines. Beside activated complement proteins,4 classical stimuluses for expression of cytokine genes are bacterial endotoxin, hypoxia,5, 6 and cytokines themselves.6
Cytokines are small, non-constitutive, peptides that can be produced by virtually all nucleated cells. They have important physiological functions, as they regulate intercellular interactions and cell growth. As far as inflammation is concerned, and based on their biological properties, cytokines can be divided into pro- and anti-inflammatory groups.7, 8
Pro-inflammatory cytokines have biological properties that relate to the stimulation of leukocytes, endothelial cells and virtually all parenchymatous cells, causing them to produce other inflammatory mediators, cytotoxicity, and initiation of cell death.7, 9 Cell death, in both its necrotic and apoptotic forms, will be discussed in detail later. Tumour necrosis factor-α and interleukin-1β, are so-called early pro-inflammatory cytokines because they are immediately up-regulated in response to an inflammatory stimulus. They initiate systemic inflammation and, because of their pyrogenic properties, they elicit the febrile response.1, 10 Interleukin-6 is another important pro-inflammatory cytokine, as it is the principal regulator of the acute phase response.1 It stimulates the intrahepatic synthesis of acute phase proteins, such as C-reactive protein. This is a central step for the termination of the systemic inflammatory reaction, since acute phase proteins possess anti-inflammatory properties.11
The natural anti-inflammatory response to an injury is mediated by anti-inflammatory cytokines, such as interleukin-10 and the interleukin-1 receptor antagonist. The appearance of these cytokines in the circulation is normally delayed compared to that of pro-inflammatory mediators, from which their synthesis, in part, depends. Interleukin-6, in later states of inflammation, also has anti-inflammatory properties, since it up-regulates interleukin-1ra and interleukin-10.12, 13
Anti-inflammatory cytokines inhibit the synthesis of pro-inflammatory cytokines.14 By activating the hypothalamo-pituitary-adrenal axis, they stimulate release of cortisol, and thence stop the systemic inflammatory response.2, 12 The principal mechanisms involved in the initiation and termination of the acute systemic inflammatory response are shown in Figure 2, whilst Table 2 summarises the biological properties of the principal cytokines involved in systemic inflammation.

Figure 2. Upon inflammatory stimulation, nucleated cells produce mediators to which belong the pro-inflammatory cytokines interleukin-1β, tumor necrosis factor-α and interleukin-6. These cytokines are responsible for the development of the systemic inflammatory reaction. By inducing the acute phase response in the liver, and the synthesis of anti-inflammatory cytokines such as interleukin-10 and interleukin-1 receptor antagonist, pro-inflammatory cytokines limit their own production. Interleukin-10 activates the hypothalamo-pituitary-adrenocortical axis and leads to release of cortisol, which in turn contributes to terminate the inflammatory process.
Table 2. Biological properties of the main pro- and anti-inflammatory cytokines involved in the pathophysiology of inflammation in the setting of cardiac surgery.

Nuclear factor kappa B plays a central role in inflammation, as it is the main transcription factor for inflammatory mediators, including pro-inflammatory cytokines such as tumour necrosis factor-α, interleukin-1β, and interleukin-6, chemokines such as interleukin-8, inflammatory enzymes such as inducible nitric oxide synthase, inducible cyclooxygenase, 5-lipoxygenase, and phospholipase A2, and adhesion molecules such as intercellular adhesion molecule-1, vascular-cell adhesion molecule-1, and E-selectin.
Nuclear factor kappa B is a heterodimer comprising two subunits, p50 and p65. In the normal state, it is bound to its natural inhibitory protein, inhibitory kappa B, which prevents it entering the nucleus.6 Upon stimulation, inhibitory kappa B becomes phophorylated and degraded. Nuclear factor kappa B is then allowed to translocate into the nucleus, where it regulates the transcription of inflammatory proteins.6 Since many inflammatory proteins controlled by Nuclear factor kappa B act as activators of this transcription factor, there is an amplifying loop of inflammation (Fig. 3).

Figure 3. Nuclear factor kappa B is the main transcription factor of inflammatory genes. Under normal conditions, nuclear factor kappa B, a heterodimer consisting of two sub-units, p50 and p65, is bound to its inhibitory protein inhibitory kappa B. Upon stimulation, this inhibitory protein undergoes phophorylation, and thereby degradation. Nuclear factor kappa B is then allowed to translocate into the nucleus, where it regulates the transcription of inflammatory genes such as pro-inflammatory cytokines, adhesion molecules, and the inducible nitric oxide synthase. Since the proteins resulting from the activity of nuclear factor kappa B are in turn its activators, an amplifying loop ensues that may lead to uncontrolled inflammation.
The inflammatory damage to tissues involves complex mechanisms characterised by the activation of leukocytes, platelets, endothelial, and parenchymal cells.15
Independent of the local or systemic character of inflammation, activated complement proteins, and pro-inflammatory cytokines, lead to the synthesis of adhesion molecules by activating Nuclear factor kappa B.6 Adhesion molecules act to increase leukocyte-endothelium interactions in a 3-stage process. First, due to the expression of E-(endothelial) and L-(leukocyte)-selectins, leukocytes move slowly on the endothelium and begin to roll on it. In the second phase, integrins such as the complement receptor-3 (CD11b/CD18) expressed on neutrophils lead to the firm adhesion of the leukocytes by binding activated adhesion molecules such as intercellular adhesion molecule-1 on endothelial cells. This step is related to leukocyte degranulation and respiratory burst, and thereby to the release of cytotoxic enzymes which damage the endothelium and the surrounding tissues. In the third stage, the activated neutrophils transmigrate into the interstitial tissues,15, 16 where they release radical oxygen species, nitric oxide, inflammatory enzymes and newly synthesised cytokines, all of which act on parenchymal cells, leading to damage and death of the cells by necrosis or apoptosis (Fig. 4).

Figure 4. The inflammatory damage to tissues is related to the activation of leukocytes by circulating mediators such as activated complement proteins and pro-inflammatory cytokines. Activated leukocytes express adhesion molecules (green arrow), which are necessary to achieve interactions between leukocytes and endothelial cells. This results in a decrease in the velocity of leukocytes in the blood vessel, in firm adhesion of leukocytes onto the endothelium, and finally in transmigration of leukocytes into the interstitial tissue, where they release toxic degranulation products, which in turn are responsible for damage to and death of cells.
From the stance of inflammation itself, it is crucial to differentiate these two different pathways for death of cells. Necrosis involves the loss of integrity of the cell membrane, and therefore is associated with the release of inflammatory products into the interstitium. It contributes to the damage of the neighbouring cells, which will also enter the pathway of death. Necrosis does not require any energy.17 Apoptosis, in contrast, is a highly genetically controlled pathway, also known as programmed cell death. Apoptosis is characterised by maintenance of the integrity of the cell membrane, and by shrinkage of the cell, which will be phagocytosed by macrophages. For this reason, apoptosis is non-inflammatory and a self-limiting form of death, which does not cause damage to neighbouring cells and tissues. Apoptosis requires energy.17, 18
Systemic or parenchymatous production of pro-inflammatory cytokines, such as tumor necrosis factor-α is related to vasodilation, increased vascular permeability, interstitial oedema, and micro- infarctions due to leukocyte plugging. These activities lead to hypoperfusion of the tissues and loss of the function of the organ.19–21
As far as the myocardium is concerned, tumor necrosis factor-α acts as a cardiodepressant cytokine. It leads to disruption in the handling of calcium, desensitises the myofilaments to calcium, and decreases β-adrenoceptor responsiveness.9 The depression of cardiac function in response to tumor necrosis factor-α is biphasic. The immediate contractile dysfunction following administration of tumour necrosis factor-α is mediated by sphingosine, a phospholipid metabolite, while the delayed and sustained systolic and diastolic dysfunction is the result of the activation of inducible nitric oxide synthase. This leads to the production of high, non-physiological, concentrations of nitric oxide.9
Natural anti-inflammatory cytokines, such as interleukin-10, play a central role in the control of systemic inflammation. Protection of organs is likely to depend on the aptitude to produce anti-inflammatory cytokines in response to inflammatory stress.12 Indeed, in the setting of sepsis, a higher ratio of the circulating levels of interleukin-6 to interleukin-10 is a predictor of poor outcome.22 Conversely, in infants undergoing cardiac surgery, a higher ratio between circulating levels of interleukin-10 and interleukin-6 is associated with less postoperative morbidity.23 Our own experimental data clearly indicate that greater production of interleukin-10 during cardiac surgery is associated with protection of the organs.24, 25
The potential for the anti-inflammatory cytokine response is probably multifactorial. Factors influencing this potential are:
While an adequate anti-inflammatory reaction is necessary to control systemic inflammation, the question as to whether excessive levels of interleukin-10 after cardiac surgery could harm by initiating immune paralysis, therefore increasing the risk of postoperative infection, has yet to be answered.33 Indeed, in patients with severe sepsis or trauma, it has been shown that an hypo-inflammatory state follows the early phase of uncontrolled inflammation. This hypo- inflammatory state is related to deactivation of monocytes, with decreased activation of nuclear factor kappa B. Although the circulating levels of interleukin-10 do not correlate with the degree of monocytic activation, they still remain elevated up to 10 days after the acute inflammatory event, suggesting that sustained production of anti-inflammatory cytokine is, at least in part, responsible for the immune paralysis observed.34, 35 This points out the importance of understanding the exact mechanisms regulating the inflammatory balance, permitting both the control of the systemic inflammation and maintenance of immune competence.
More than twenty years ago, Chenoweth and his group36 first reported the activation of the complement system during cardiopulmonary bypass in adults. Two years later, Kirklin37 demonstrated the positive relationship between activation of complement and postoperative morbidity in adults and in children. Since then, great efforts have been made to define better the mechanisms involved in the systemic inflammatory reaction associated with cardiac surgery. It is widely accepted today that the contact between blood and foreign surfaces during cardiopulmonary bypass initiates the activation of the alternative pathway of the complement system.36–38 At the end of cardiopulmonary bypass, activation of complement is enhanced by heparin-protamine complexes that act on the classical pathway.39
The complement system has many interactions with the contact system of the plasma, with the coagulation system, and with the system of fibrinolysis. All of them are activated in the setting of cardiac surgery.40 Together, they contribute to sustain systemic inflammation. Activated complement proteins influence leukocytic function by stimulating mobilisation, adhesion, and degranulation of the leukocytes, and also synthesis of cytokines.6, 41 The principal mechanisms involved in the pathophysiology of cardiopulmonary bypass are shown in Figure 5.

Figure 5. Cardiac operations involving cardiopulmonary bypass are associated with a systemic inflammatory reaction due, in the first instance, to the contact between blood and artificial surfaces, resulting in activation of the complement system. This is enhanced by ischemia followed by reperfusion of the organs, and by the activation of the Hageman system, along with the coagulative and the fibrinolytic cascades. Activated complement proteins, ischemia, and reperfusion are among others inducers of leukocytic activation and synthesis of pro-inflammatory cytokines, which contribute to the synthesis of anti-inflammatory cytokines.
Neonates and young children undergoing cardiac surgery with cardiopulmonary bypass show significant C3- and C5-conversion,37, 42–44 indicating late phase activation of the complement system up to the terminal membrane attack complex. Significant activation of complement is observed as early as 10 minutes after institution of cardiopulmonary bypass, and the peak value of circulating activated complement proteins is observed at the end of cardiopulmonary bypass.43 By this time, several simultaneous events have taken place, including the reinstitution of myocardial perfusion, the rewarming of the patient, and the administration of protamine, all factors that sustain activation of complement.39, 45, 46
The importance of leukocytic activation in causing damage to the tissues during cardiac surgery in children is underscored by studies showing improved myocardial function in piglets treated with an inhibitor of Complement receptor-3 (CD11b/CD18) up-regulation on leukocytes.47 In addition to that, the magnitude of release of elastase or histamine from activated leukocytes observed during cardiac surgery in neonates and children has clearly been associated with the development of postoperative complications.43, 48
Neonates, infants and children undergoing cardiac operations with cardiopulmonary bypass show significant production of pro-inflammatory cytokines, such as tumor necrosis factor-α interleukin-6, and interleukin-8.49 There is also a significant production of interleukin-10,50, 51 indicating the potential for the anti-inflammatory response. Usually, concentrations of tumour necrosis factor-α, interleukin-6, and interleukin-8 reach maximal values in the plasma at the end of cardiopulmonary bypass, while those of interleukin-10 are measured either at the end of cardiopulmonary bypass,52 or later on the first postoperative day.50 The precise time depends on the perioperative management, including among others, pretreatment with corticosteroids,30 and the degree of hypothermia achieved during cardiopulmonary bypass.50
Complement anaphylatoxins,41 and endotoxin released from the gut,53 are thought to be the major triggers for pro-inflammatory production of cytokines during cardiac surgery in children. Other mechanisms, such as hypoxia and reoxygenation,54 and operative trauma,55 probably also play an important role.
While a certain degree of inflammation is probably necessary to stimulate angiogenesis, to permit repair of tissue and healing of wounds after cardiac surgery, and to protect the patient from infection during the stay in hospital, there is no doubt that excessive and uncontrolled inflammation is harmful, especially in very young children.43, 48
Unfortunately, there is no consensus on the adequate definition of the systemic inflammatory reaction syndrome and inflammation-related complications after cardiac surgery in the children. Reliable epidemiological data, therefore, are not available. Nevertheless, postoperative dysfunction of organs associated with uncontrolled inflammation has been reported in children, and recognised as a significant problem carrying a high mortality.43
The capillary leak syndrome is a common feature after cardiac surgery in children. It is characterised by shift of fluid into the interstitial space, with the development of generalised oedema, pleural effusion, and ascites.56 If unrecognised and untreated, the hypovolemic state leads to hypoperfusion of the tissues, and damage to organs. Neonates are particularly at risk of developing the capillary leak syndrome, because they have increased movement of fluid across the capillary membrane, leading to rapid shifts from the intra-vascular to the extra-vascular space. In addition, they have some degree of lymphatic insufficiency.57
In neonates undergoing cardiac surgery, factors influencing the postoperative development of the capillary leak syndrome include a preoperative inflammatory state, characterised by activation of complement and leukocytosis, and an enhanced inflammatory response to cardiac surgery, with increased release of histamine and tumour necrosis factor-α.
The increased capillary permeability produced by the endothelial damage is the substrate for damage to organs and their dysfunction, as shown in an experimental model of cardiac surgery.24 As far as the myocardium is concerned, a clear association exists between systemic and intra-parenchymatous synthesis of tumor necrosis factor-α and the degree of intracellular and interstitial oedema due to the rupture of the cell membrane.25
The association between the production of pro-inflammatory cytokines and the development of myocardial cellular damage in neonates has recently been reported by our group.58 Indeed, while cardiac surgery with cardiopulmonary bypass causes significant production of interleukin-6 and interleukin-8 in all neonates undergoing the arterial switch operation, those who develop postoperative myocardial dysfunction have higher circulating levels of pro-inflammatory cytokines than do those without myocardial dysfunction. The correlation between postoperative levels of cytokines and troponin-T suggest a causative relationship between inflammation and myocardial cellular damage.
We recently demonstrated this causative relationship in an animal model.25
Mediators such as histamine not only have inflammatory properties, but also act as potent arrhythmogenic substances.59 Histamine is released during cardiopulmonary bypass from mast cells and circulating basophil leukocytes, both targets for a large number of inflammatory mediators such as complement anaphylatoxins and cytokines. Histamine increases sinus rate, produces various degrees of atrioventricular block, and enhances the automaticity of ectopic pacemakers.60 A large number of experimental studies have demonstrated that histamine induces sinus tachycardia, junctional ectopic tachycardia, and ventricular tachycardia, and that these effects are mediated through its H2-receptors.59
Transient postoperative arrhythmias, such as junctional ectopic tachycardia, which classically occur after cardiac surgery involving cardiopulmonary bypass, are serious complications of surgery for congenital cardiac defects.61 Junctional ectopic tachycardia is particularly poorly tolerated in patients with reduced diastolic function, as it leads to reduced ventricular filling, and to the loss of the sequential atrioventricular activity.
We suggested that the development of transient arrhythmias, mainly junctional ectopic tachycardia, after cardiac surgery in children could be related to intraoperative liberation of histamine.48 We also confirmed the relationship between systemic inflammation and the occurrence of accelerated nodal rhythms, including junctional ectopic tachycardia, after cardiac surgery. Indeed, in a consecutive cohort of 113 children undergoing cardiac surgery with cardiopulmonary bypass, concentrations of interleukin-6 were measured in the blood, and interpreted as a marker of systemic inflammation. The 33 patients who developed accelerated nodal rhythms had higher levels of interleukin-6 immediately after cardiopulmonary bypass and 4 hours postoperatively than did the 80 children who did not show this complication (Fig. 6).

Figure 6. Plasma concentrations of interleukin-6 measured at the end of cardiopulmonary bypass and 4 hours postoperatively in 113 infants and children having undergone cardiac surgery. Concentrations of interleukin-6 are significantly higher in the 33 patients with accelerated junctional rhythm (yellow) than in the 80 without this complication (white). Results are shown as mean ± standard error of the mean; *p < 0.05 between patients with or without accelerated junctional rhythm (Mann-Whitney test).
Prospective randomised trials should be undertaken to test the hypothesis that histamine antagonists, such as the blockers cimetidine or ranitidine, the latter showing structural similarity with amiodarone,62 would be efficient and safe for the prevention of rapid nodal rhythms after cardiac surgery in children.
Since inflammatory mediators potentially target all circulating and parenchymatous cells, an uncontrolled systemic inflammatory reaction will manifest itself clinically as multiple failure of organs, a syndrome observed in the setting of sepsis, trauma, and major surgery.63 Cardiac operations with cardiopulmonary bypass are representative of this latter category.
In a study designed to analyse the relationship between the systemic inflammatory reaction related to cardiac surgery and the development of postoperative failure of multiple organs, activation of complement, and release of leukocyte elastase were higher in children who developed organ failure than in the others.43 Patients with failure of multiple organs all had acute renal and hepatic failure, in addition to cardiovascular insufficiency, thrombocytopenia, disorders of coagulation, and temperatures higher than 39 degrees centigrade. Most of them also had transient arrhythmias such as junctional ectopic tachycardia, respiratory insufficiency, and neurological morbidity. In accordance with the high mortality known to be related to the failure of multiple organs, one-third of our patients with this syndrome died postoperatively.
There is, as yet, no specific treatment of the uncontrolled systemic inflammatory response syndrome. A modulation of this response, nonetheless, can be achieved by appropriate pharmacological and technical strategies during the perioperative management. These strategies are aimed at enhancing the balance in favour of the anti-inflammatory response to cardiac surgery, either by decreasing the production of pro-inflammatory mediators or removing them from the circulation, or by enhancing the production of the anti-inflammatory mediators.
Since the primary force of the systemic inflammatory reaction elicited by cardiac surgery is activation of complement,64 it is tempting to inhibit the complex cascade of events which ensues at its first step. This can be achieved by nonspecific or specific inhibitiors of the complement system.
Donors of nitric oxide, such as sodium nitroprusside, have been shown experimentally to possess properties that inhibit complement, probably by inhibiting C3-conversion.65 In a previous study, we showed that children given low doses of sodium nitroprusside, 1 μg/kg body weight/minute, during cardiopulmonary bypass had lower degrees of activation of complement during and after cardiac surgery, and also lower postoperative morbidity, than those who were not treated in this fashion.65
The inhibitor of C1-esterase controls the early phase of the activation of the classical pathway of the complement system, and also inhibits the Hageman factor, coagulation factor X, and fibrinolysis.41 Although we did not find any difference in the concentrations of the inhibitor in neonates with and without capillary leak syndrome,56 Tassani and collaborators reported improved pulmonary function in patients receiving the inhibitor before the operation.66 Due to its antifibrinolytic properties, which could enhance the potential for postoperative thrombosis, the inhibitor of C1 esterase should be utilised with caution in small infants.
The recombinant soluble complement receptor type 1, which inhibits the binding of complement fragment C3 to its receptor, might be useful in mitigating the systemic inflammatory response after cardiac surgery.67
Heparin-coated circuits have been shown to increase biocompatibility by attenuating the activation of complement and stimulation of neutrophils.68 Few open trials have suggested that they might improve output of urine and oxygenation in the early postoperative period.69 Postoperative morbidity and mortality, however, are not significantly reduced.70
Serine protease inhibitors such as aprotinin have been claimed to decrease the inflammatory response to cardiac surgery in adults.71 This effect, as yet, has not been confirmed in children.72
Vitamin C and N-acetylcysteine act as scavengers of free radicals. As such, they might decrease the neutrophil oxidative burst during cardiac surgery.73, 74
Ultrafiltration, introduced by Elliott 10 years ago,75 has now become standard in the perioperative management of children undergoing cardiac surgery. In addition to its beneficial effects in removing fluids, which are associated with improved hemodynamics, ultrafiltration is also thought possibly to remove inflammatory mediators from the circulation.51 Peritoneal drainage after cardiac surgery in neonates could also provide dialysis of pro-inflammatory cytokines.76
Glucocorticosteroids inhibit the synthesis of all inflammatory mediators having nuclear factor kappa B as their transcription factor (see above). Basically, glucocorticosteroids act by binding glucocorticoid response elements on glucocorticoid-responsive genes, thereby increasing the transcription of many anti-inflammatory proteins such as interleukin-10, interleukin-1ra, Lipocortin-1, and inhibitory kappa B.6 This effect is genomic. Glucocorticoids also repress the expression of many inflammatory genes by inhibiting nuclear factor kappa B.6 This effect is non-genomic.
In adults, glucocorticoids administered before cardiac surgery blunt the production of tumor necrosis factor-α and interleukin-8, and increase that of interleukin-10.30 In children, the administration of dexamethasone at 1mg/kg body weight reduces the production of tumour necrosis factor-α and interleukin-6, and produces a substantial improvement in outcome.77
Moderate hypothermia during cardiopulmonary bypass has been suggested by us to increase the production of interleukin-10 in infants.50 This observation was confirmed in an animal model of cardiac surgery, where cooling the circulating blood down to 28 degrees centigrade was associated with increased production of interleukin-10 in blood and parenchymal cells, and with decreased synthesis of tumour necrosis factor-α.24 This shift of the inflammatory response toward a net anti-inflammatory reaction was associated with fewer cells dying, and with greater protection of organs.24, 25
As stated above, the potential to initiate and to control the systemic inflammatory response depends on individual factors, in which is included the preoperative condition. During cardiac surgery in children, preoperative conditions potentially affecting the systemic inflammatory response to cardiac surgery, in addition to preoperative infection, mainly involve myocardial hypertrophy, myocardial failure, hypoxaemia, or a combination of these factors.23
Haemodynamic overload, such as intracardiac left-to-right shunting leading to congestive heart failure or hypoxaemia, stimulates production of nuclear factor kappa B, and therefore induces the pro-inflammatory cytokines.78, 79 Circulating levels of interleukin-6 are abnormally elevated in infants with hypoxaemia or heart failure scheduled for primary cardiac surgery, the former having the highest concentrations that correlate inversely with preoperative arterial saturations of oxygen.23 After the operation, levels of interleukin-6 remain higher in patients with preoperative hypoxaemia, indicating a priming of immune competent cells by hypoxaemia that is possibly responsible for the enhanced inflammatory response to cardiac surgery seen in those patients.23 The atrial and the ventricular myocardium of infants requiring cardiac surgery with cardiopulmonary bypass also contain substantial amounts of cytokines, which could well increase intramyocardial inflammation in response to surgery, and therefore contribute to perioperative myocardial damage.58
Cardiac surgery involving cardiopulmonary bypass in children is related to a systemic inflammatory response that comprises activation of complement, stimulation and degranulation of leucocytes, synthesis of cytokines, and increased interactions between the leucocytes and the endothelium. Contact between blood and foreign surfaces during cardiopulmonary bypass, and ischemia-reperfusion injury, are probably the main inductors of this complex reaction of the organism, primarily acting as a mechanism of homeostasis. Due to reasons as yet unclarified, the systemic inflammatory reaction can become uncontrolled, and then becomes the direct cause of specific postoperative morbidity. Complications, such as the capillary leak syndrome, the multiple organ dysfunction syndrome, and certain types of transient postoperative arrhythmias, are examples for this in neonates and young children.
Technical and pharmacological interventions aimed at modulating, but not completely suppressing, the systemic inflammatory response occurring during cardiac surgery are promising in providing substantial protection for the organs.
Besides the further clarification of the pathophysiological mechanisms contributing to the systemic inflammatory response to cardiac surgery, modulation of the balance between pro- and anti-inflammatory mediators, and stratification of the risk in our population of patients, together with excellent operative and standard postoperative treatment, are mandatory for the achievement of very low postoperative morbidity.

The complement cascade can be activated throughout either the classical or the alternative pathways. The activation of the classical pathway begins with the binding of an activator to C1, which is composed of 3 portions C1q, C1r and C1s. C1 activates in turn C2 and C4, which are split into C2a, C2b and C4a, C4b, respectively. The split products C2a and C4b bind to form the C3-convertase of the classical pathway.The activation of the alternative pathway probably depends upon cleavage of small amounts of C3 in C3a and C3b. C3b is then available for binding factor B. Factor D cleaves bound factor B to produce Ba and Bb. The complex C3bBb is the C3-convertase of the alternative pathway that is stabilised by factor P, also called properdine.Both C3-convertases cleave C3 into C3a and C3b. By binding an additional C3b, both C3-convertases become C5-convertases. The cleavage of C5 produces C5a and C5b. C5b bounds C6 and in a stepwise fashion, C7, C8 and C9 are added into the complex to form the so called membrane attack complex. The main molecules with biological properties are represented in red (anaphylatoxins) and in blue (membrane attack complex).

Table 1.

Upon inflammatory stimulation, nucleated cells produce mediators to which belong the pro-inflammatory cytokines interleukin-1β, tumor necrosis factor-α and interleukin-6. These cytokines are responsible for the development of the systemic inflammatory reaction. By inducing the acute phase response in the liver, and the synthesis of anti-inflammatory cytokines such as interleukin-10 and interleukin-1 receptor antagonist, pro-inflammatory cytokines limit their own production. Interleukin-10 activates the hypothalamo-pituitary-adrenocortical axis and leads to release of cortisol, which in turn contributes to terminate the inflammatory process.

Table 2.

Nuclear factor kappa B is the main transcription factor of inflammatory genes. Under normal conditions, nuclear factor kappa B, a heterodimer consisting of two sub-units, p50 and p65, is bound to its inhibitory protein inhibitory kappa B. Upon stimulation, this inhibitory protein undergoes phophorylation, and thereby degradation. Nuclear factor kappa B is then allowed to translocate into the nucleus, where it regulates the transcription of inflammatory genes such as pro-inflammatory cytokines, adhesion molecules, and the inducible nitric oxide synthase. Since the proteins resulting from the activity of nuclear factor kappa B are in turn its activators, an amplifying loop ensues that may lead to uncontrolled inflammation.

The inflammatory damage to tissues is related to the activation of leukocytes by circulating mediators such as activated complement proteins and pro-inflammatory cytokines. Activated leukocytes express adhesion molecules (green arrow), which are necessary to achieve interactions between leukocytes and endothelial cells. This results in a decrease in the velocity of leukocytes in the blood vessel, in firm adhesion of leukocytes onto the endothelium, and finally in transmigration of leukocytes into the interstitial tissue, where they release toxic degranulation products, which in turn are responsible for damage to and death of cells.
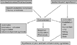
Cardiac operations involving cardiopulmonary bypass are associated with a systemic inflammatory reaction due, in the first instance, to the contact between blood and artificial surfaces, resulting in activation of the complement system. This is enhanced by ischemia followed by reperfusion of the organs, and by the activation of the Hageman system, along with the coagulative and the fibrinolytic cascades. Activated complement proteins, ischemia, and reperfusion are among others inducers of leukocytic activation and synthesis of pro-inflammatory cytokines, which contribute to the synthesis of anti-inflammatory cytokines.

Plasma concentrations of interleukin-6 measured at the end of cardiopulmonary bypass and 4 hours postoperatively in 113 infants and children having undergone cardiac surgery. Concentrations of interleukin-6 are significantly higher in the 33 patients with accelerated junctional rhythm (yellow) than in the 80 without this complication (white). Results are shown as mean ± standard error of the mean; *p < 0.05 between patients with or without accelerated junctional rhythm (Mann-Whitney test).