Paediatric cardiac transplantation has become increasingly common in patients with end-stage cardiac disease. Outcomes for children transplanted for CHD have been demonstrated to be distinctly different compared to cardiomyopathy patients.Reference Dipchand1– Reference Everitt, Boyle and Schechtman3 At 3 years post-transplant, survival was 88 and 79% in cardiomyopathy and CHD recipients, respectively.Reference Rossano, Dipchand and Edwards4 Despite studies emphasising the discrepancies between these populations pre- and post-transplant, the mechanisms behind these difference in outcomes are incompletely understood. To better understand the potential differences between these two patient groups, we sought to use cardiopulmonary exercise testing to assess for differences in graft function that may not be apparent on resting assessment.
Differences measured during cardiopulmonary exercise testing between these populations may offer some insight into the divergences observed in outcomes. In adult heart transplant populations, cardiopulmonary exercise testing is used frequently to assess overall exercise capacity, as part of evaluation to for coronary artery disease, and for predicting cardiovascular mortality.Reference Myers, Tan, Abella, Aleti and Froelicher5– Reference Chaudhry, Kumar and Behbahani7 Further, studies have also shown that peak oxygen uptake and self-reported physical health are strong predictors of long-term survival in adult heart transplant patients.Reference Yardley, Havik, Grov, Relbo, Gullestad and Nytroen8 Similar studies have been performed in paediatric heart transplant patients but are limited and show inconsistent results.Reference Davis, McBride, Chrisant, Patil, Hanna and Paridon9– Reference Dipchand, Manlhiot, Russell, Gurofsky, Kantor and McCrindle12 Previous paediatric studies have not assessed the differences in cardiopulmonary exercise testing between children with a primary diagnosis of CHD or cardiomyopathy.
The aim of this study was to assess exercise capacity using cardiopulmonary exercise stress testing in post-transplant patients grouped by primary underlying diagnosis leading to heart transplantation, CHD versus cardiomyopathy. We hypothesised that patients with CHD would have differences in graft function that could be evaluated with cardiopulmonary exercise testing given their higher rate of mortality post-transplant.
Methods
The methodology for this study was reviewed and approved by the Institutional Review Board at our institution. The methodology for this study was also ensured to be compliant with the Declaration of Helsinki.
Patients having undergone heart transplant at our institution between 1999 and 2015 were identified from the clinical transplant database. All patients in the transplant database were then cross-referenced with the clinical database for the cardiopulmonary exercise testing laboratory. Patients were included for final analysis if they had undergone at least one post-transplant cardiopulmonary exercise test 6 months or more after their transplantation date. This 6-month buffer was added to prevent confounding from deconditioning in the post-transplant period. If multiple cardiopulmonary exercise tests were performed, then the most recent test was used with correlating clinical data. Only patients who were able to cooperate with maximal effort during cardiopulmonary exercise test based on patient-perceived effort and RQ value were included.
Clinical and laboratory data were collected for all patients included in the final analysis. Transplant-related parameters that were collected included the indication for transplant, history of biopsy-proven rejection, angiographic coronary artery graft vasculopathy, and need for retransplantation. Additional clinical information including diagnoses of lung disease, plastic bronchitis, and kidney disease was recorded. Patients underwent testing via the Bruce protocol or modified Bruce protocol on a T-2100 treadmill ergometer (GE Healthcare, Wauwatosa, Wisconsin, United States of America). A 12-lead ECG rhythm strip was performed during testing for heart rate and arrhythmia analysis. During this protocol, blood pressure readings were taken at the end of each stage, and minute-to-minute breath ventilations were measured throughout testing (Encore 29c, VMAX, Palm Springs, California, United States of America). Patients were encouraged to exercise to the point of exhaustion, with test termination for fatigue, pain, or concerning arrhythmia. Chronotropic incompetence was defined in this study as the inability to achieve equal to or greater than 80% of predicted heart rate peak HR for age. Predicted peak heart rate was defined as 220 beats per minute (bpm) – age (in years).
Catheterisation data and echocardiographic data were collected for included patients. Catheterisation data were only collected and analysed if the catheterisation occurred within 6 months of the cardiopulmonary exercise testing. Parameters that were collected included the right ventricular end diastolic pressure, pulmonary artery capillary wedge pressure, pulmonary vascular resistance, and cardiac index. Cardiac index was calculated by thermodilution. Echocardiographic data were collected from the echocardiogram done within 6 months of the analysed cardiopulmonary exercise test. Parameters that were collected included ejection fraction and peak tricuspid valve regurgitant jet velocities.
Patients were segregated into two groups for analysis based on the primary indication for heart transplant: CHD or cardiomyopathy. All collected endpoints were then compared between the two groups. Descriptive variables were compared using a chi-squared analysis while continuous variables were compared using a student t-test, continuous variables were reported as mean + standard deviation (SD). These particular statistical tests were selected based on the normalcy of the data. All statistical analyses were conducted using SPSS Version 23.0. A p-value of less than 0.05 was considered statistically significant.
Results
Study cohort
A total of 35 patients who underwent at least one maximal effort cardiopulmonary exercise test at least 6 months after cardiac transplant were included in the final analyses. Two patients were excluded due to submaximal effort.
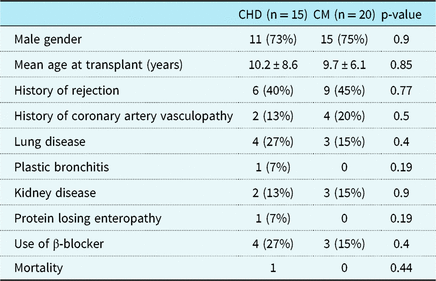
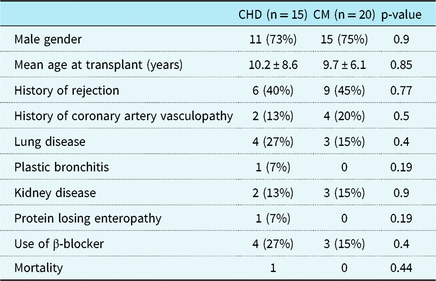
Of these, 15 were transplanted for CHD (double-inlet left ventricle n = 4, hypoplastic left heart syndrome n = 6, transposition of the great arteries n = 3, and tetralogy of Fallot n = 2); 20 were transplanted for cardiomyopathy (dilated n = 17 and restrictive n = 3). Prevalence of comorbidities, including pulmonary, renal, and hepatic disease, was similar in both groups. Mortality was 1 (6.7%) in the CHD group and 3 (15%) in the cardiomyopathy group (p = 0.44) (Table 1).
Table 1. Clinical characteristics of the 35 patients
CHD = congenital heart disease; CM = cardiomyopathy
Age at transplant was not significantly different between the two groups with the mean age of transplant being approximately 10 years in both groups (CHD 10.2 ± 8.6 years; 9.7 ± 6.1 years for cardiomyopathy). There were no differences in prevalence of male gender between the groups, with 11 male (73%) in CHD group and 15 males (75%) in the cardiomyopathy group (p = 0.91).
Time from transplant to cardiopulmonary exercise testing also did not differ between the groups with a mean of 7.03 ± 5.6 years in the CHD group and mean of 6.06 ± 5.9 years in the cardiomyopathy group (p = 0.9).
At least a single episode of rejection was documented between transplant and cardiopulmonary exercise testing in six (40%) of CHD patient and nine (45%) of those with cardiomyopathy (p = 0.95). Need for retransplant was similar in both groups at 6.7% in the CHD group and 5% in the cardiomyopathy group (p = 0.67) (Table 1).
Echocardiography
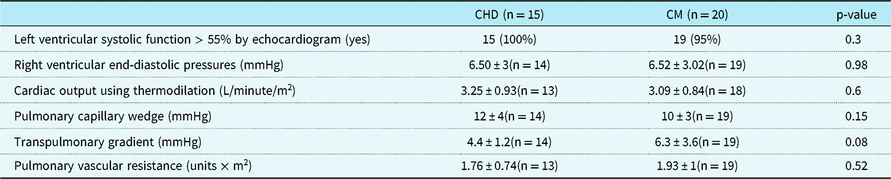
Left ventricular ejection fraction was normal (defined as left ventricular ejection fraction of ≥ 55%) in 100% of CHD patients and 95% of cardiomyopathy patients. Diastolic measurements were not routinely analysed and therefore not be reported.
Cardiac catheterisation
Catheterisation data demonstrated no significant differences between the two groups. Right ventricular end diastolic pressure (CHD 6.5mmHg ± 3 (n = 14), cardiomyopathy 6.52mmHg ± 3.02 (n = 19); p = 0.98), pulmonary capillary wedge pressure (CHD 12mmHg ± 4 (n = 14), cardiomyopathy 10mmHg ± 3 (n = 19); p = 0.15), pulmonary vascular resistance (CHD 1.76 units × m2 ± 0.74 (n = 13), cardiomyopathy 1.93 units × m2 ± 1 (n = 19); p = 0.52), and cardiac index (by thermodilution). The CHD group had a transpulmonary gradient of 4.4mmHg ± 1.2 compared to a transpulmonary gradient of 6.3 ± 3.6 in the cardiomyopathy group (p = 0.08) (Table 2).
Table 2. Echocardiographic and cardiac catheterisation data within 6 months of CPET
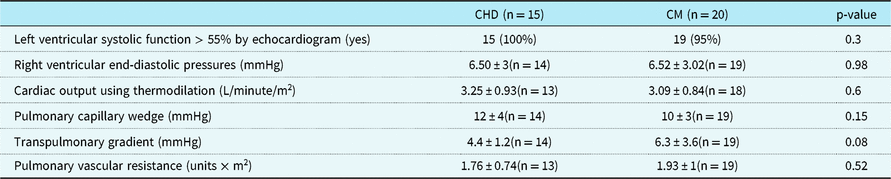
CHD = congenital heart disease; CM = cardiomyopathy; CPET = cardiopulmonary exercise test
Right ventricular end-diastolic pressures, cardiac output (using thermodilution technique), pulmonary capillary wedge, and transpulmonary gradient were all obtained during cardiac catheterisation; pulmonary vascular resistance was calculated by the hemodynamic data from the cardiac catheterisation
Cardiopulmonary exercise testing
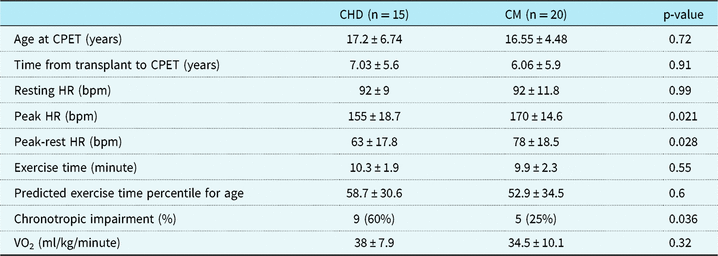
Absolute exercise duration and exercise duration percentile did not significantly differ between the groups (p = 0.55). Both groups had below than exercise time percentile for age (CHD 58.7 percentile ± 30.6, cardiomyopathy 52.9 ± 34.5); however, between the two groups there was not a difference (p = 0.6). Resting heart rate was 92 bpm ± 9 in the CHD disease group compared to 92 bpm ± 11.8 in the cardiomyopathy group (p = 0.97). Peak heart rate during cardiopulmonary exercise testing was significantly different between the two groups with a peak heart rate of 155 bpm ± 18.7 in the CHD group and 170 bpm ± 14.6 in the cardiomyopathy group (p = 0.02). Maximum oxygen consumption (ml/kg/minute) was measured in 14 of the 15 CHD patients and in 16 of the 20 cardiomyopathy patients. The mean maximum VO2 was 38 ml/kg/minute in the CHD group and 34.5 ml/kg/minute for the cardiomyopathy group (p = 0.32) (Table 3).
Table 3. CPET results and chronotropic impairment, which is defined as inability to achieve less than or equal to 80% of maximum predict heart rate for age (220 bpm – age (in years))
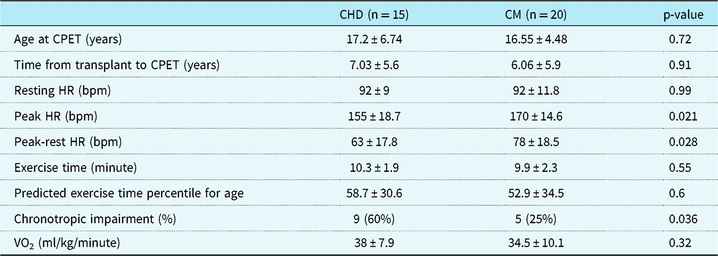
CHD = congenital heart disease; CM = cardiomyopathy; CPET = cardiopulmonary exercise test
Heart rate response was also significantly different between the two groups with a heart rate response of 63 bpm in the CHD group ± 17.8 bpm and 78 bpm in the cardiomyopathy group ± 18.5 (p = 0.03). When classifying each patient in both groups as having chronotropic incompetence as “yes” or “no” based on an inability to reach ≥ 80% of predicted heart rate for age, significantly more patients with CHD (nine patients, 60%) had chronotropic incompetence compared to cardiomyopathy patients (five patients, 25%, p = 0.04) (Table 3).
Discussion
The goal of this pilot study was to asses for differences in patients after paediatric heart transplant based on their primary diagnosis by using cardiopulmonary exercise testing, a non-invasive tool which can be performed as an outpatient evaluation. We found that patients with primary diagnosis of CHD who underwent heart transplant had diminished heart rate response and increased chronotropic incompetence as compared to those with primary diagnosis of cardiomyopathy.
Interestingly, other cardiopulmonary exercise test parameters which were measured for these patients were similar, specifically exercise time and maximum oxygen consumption. Pulmonary function testing was not routinely performed prior to the last few years with our cardiopulmonary exercise testing; therefore, differences in pulmonary function test results could not be assessed. Additional clinical post-transplant diseases, such as chronic kidney and lung disease, were also not different between the two groups.
Abnormal heart rate response and chronotropic incompetence during cardiopulmonary exercise test have been reported as risk factors for morbidity in different disease populations.Reference Liontou, Chrysohoou, Skoumas, Panagiotakos, Pitsavos and Stefanadis13– Reference Wu, Yen and Lee15 In adults with CHD, cardiopulmonary exercise testing was used to determine if chronotropic incompetence could be used as a prognostic marker. It was found that abnormal heart rate response to exercise was prevalent in over half of the adult CHD population studied and was associated with diminished exercise capacity and predicted a higher mortality risk.Reference Diller, Dimopoulos and Okonko14
Only a small subset of studies have specifically assessed cardiopulmonary exercise test parameters in paediatric heart transplant recipients; however, they have shown similar findings of decreased heart rate response and chronotropic incompetence compared to normal paediatric populations.Reference Pahl, Sundararaghavan and Strasburger16– Reference Singh, Gauvreau, Rhodes and Blume19 Giardini et al in 2013 published that a blunted heart rate response was present initially after paediatric heart transplantation, which normalised around 6 years post-transplant but then starts to again decline after this time, which correlated with a higher rate of death and retransplantation.Reference Giardini, Fenton, Derrick and Burch18 More recently, a systematic review looking at six major databases comparing cardiopulmonary exercise tests using treadmill or electronically braked cycle ergometer in children who received heart transplant to a healthy control group found that resting heart rate was higher compared to controls but that peak heart rate, heart rate reserve, peak oxygen consumption, exercise duration, and minute ventilation were significantly lower compared to controls or normative data.Reference Peterson, Su, Szmuszkovicz, Johnson and Sargent20 In our study, we were unable to compare serial cardiopulmonary exercise tests in this cohort as routine testing was not routinely performed following heart transplant; however, in the last few years, our practice has now incorporated cardiopulmonary exercise testing as an additional clinical test to monitor patients with plans for serial testing every 2–3 years.
Paediatric studies, however, do not distinguish patients based on their primary diagnosis. As more children are being transplanted for CHD, understanding the ongoing differences of these patients post-transplant remains crucial. Given the known difference in outcomes after heart transplant, between patients with cardiomyopathy and patients with CHD, it is important to understand how these groups differ and to evaluate for clinical data that may indicated higher risk for negative outcomes.
The current study is the first to directly compare cardiopulmonary exercise test results in children’s post-heart transplant based on primary diagnosis of CHD and cardiomyopathy. The reason for discrepancy in chronotropic response to exercise in our cohort is unclear. A potential explanation of these data is that patients with CHD have often undergone multiple surgeries prior to heart transplantation which may affect their ability to reinnervate their hearts’ post-transplant. Denervation is a known process associated with less heart rate variability and lower peak heart rate following heart transplantation. While evidence exists that reinnervation can occur following heart transplant in children, this process is inconsistent and can take years to occur.Reference Grupper, Gewirtz and Kushwaha21
Although we did not find a difference in incidence for rejection, coronary artery vasculopathy, or death in this initial study, ongoing prospective monitoring with serial cardiopulmonary exercise tests over time would be necessary to further differentiate long-term implications. Previously, our program did not use cardiopulmonary exercise testing as routine testing post-transplant but is now being incorporated as routine clinic evaluation, ideally every 2–3 years. Some centres already incorporate routine cardiopulmonary exercise tests or stress echocardiography protocols into their routine surveillance as a means of providing non-invasive assessment for coronary artery vasculopathy. This study demonstrates a difference in heart rate response and rates of chronotropic incompetence that may be important for normalising such analysis.
Differences in heart rate response and chronotropic incompetence could have important implications for quality of life in these patients. Heart rate response is tied to cardiac output and therefore exercise capacity. Limitations in one’s ability to increase cardiac output with exercise would be expected to impact a patient’s ability to participate in more demanding physical activities. This may ultimately cause patients to perceive their exercise capability as limited and lead them to avoid activities which could benefit their cardiovascular health.
Limitations
Limitations of this study include its retrospective nature, small sample sizes, and lack of longitudinal follow-up. Due to these limitations, caution should be used in the generalisation of results. While we did not find outcome differences in the current analysis, the current pilot study was not powered to detect these rare events, and longer-term follow-up with serial cardiopulmonary exercise tests are necessary to assess the clinical impact. While further prospective study with prolonged follow-up would be needed to show associations to long-term outcomes, the initial findings of this study do demonstrate a potentially important difference between patients transplanted for CHD versus cardiomyopathy and provide important normative data for the interpretation of cardiopulmonary exercise testing in the general follow-up of paediatric heart transplant patients.
Conclusion
Our results show that paediatric heart transplant recipients with a primary diagnosis of CHD have a lower heart rate response and increased chronotropic incompetence when compared to patients with a primary diagnosis of cardiomyopathy. Our initial pilot data highlight the ongoing physiologic dissimilarities in these two recipient cohorts even following heart transplant.
Author ORCIDs
Nikki M. Singh 0000-0002-4125-9794
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.