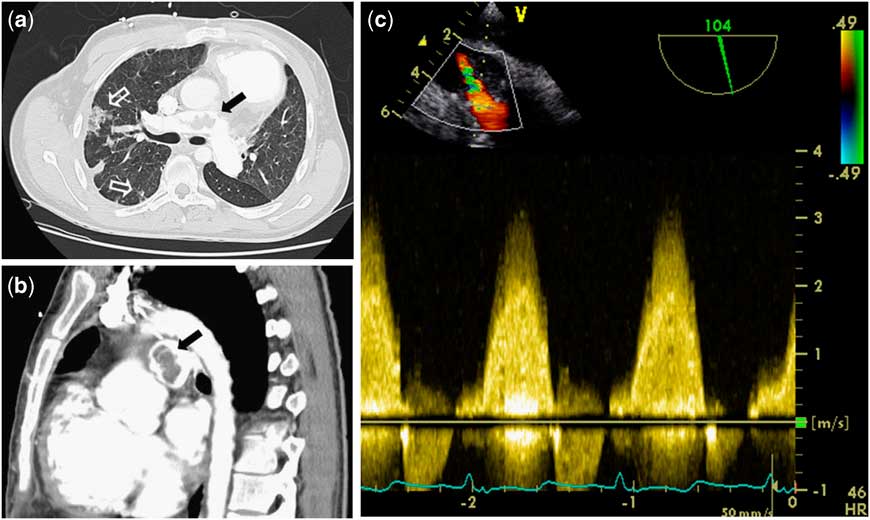
A 21-year-old adult patient with Tetralogy of Fallot presented acutely unwell with a 3-month history of general malaise following dental treatment. Following initial complete repair, he subsequently underwent two right ventricular outflow tract reconstructions with conduits. On admission, transthoracic echocardiography and peripheral blood cultures were suggestive of streptococcal endocarditis. Sequential computed tomography pulmonary angiography demonstrated peripheral emboli with progressive central pulmonary artery filling defects suggestive of thrombi and potential vegetations (Fig 1a and b). Urgent surgery was performed for uncontrolled sepsis and increasing hypoxia. Peri-operative transesophageal echocardiogram confirmed previous findings (Fig 1c; Supplementary Video 1). Having resected the conduit, we performed a peripheral pulmonary embolectomy and resected an abundance of the infected material from the pulmonary arterial tree under low-flow hypothermia; we then proceeded with a jugular venous valved conduit (Contegra®, Medtronic Inc, Minneapolis, Minnesota, United States of America) replacement and tricuspid valve annuloplasty. He made a slow but steady recovery and was discharged home in good health.

Figure 1 ( a and b ) Computed tomography pulmonary angiography demonstrating peripheral septic emboli (white arrows) with filling defects in the right main pulmonary artery (black arrows) due to purulent debris and clot as seen intra-operatively. ( c ) Transesophageal echocardiography before the fourth sternotomy clearly showing high-velocity flow at the same level.
Right ventricular outflow tract conduit endocarditis remains a rare event.Reference Dave, Mueggler and Comber 1 Prompt conduit replacement in challenging cases such as the one described remains our strategy of choice. Extra-corporeal membrane oxygenation may be utilised in the pre-operative period if cardio-respiratory failure ensues. Although we had made provision for extra-corporeal membrane oxygenation availability intra/post-operatively, this was not necessary.