Article contents
Catheter-based closure of a perimembranous ventricular septal defect using the Amplatzer™ occluder in a patient with right-sided heart and mirror-imaged arrangements of all organs
Published online by Cambridge University Press: 16 September 2005
Abstract
We describe a 6-year-old boy with a right-sided heart part of the Kartagener syndrome, complicated by presence of a perimembranous ventricular septal defect. The defect was closed interventionally using the Amplatzer™ asymmetrical occluder for ventricular septal defects. The procedure was event-free. Non-operative closure of a ventricular septal defect in this patient demonstrated no other difficulty than mirror-imaged thinking, thus giving evidence that such treatment also can be offered to patients with positional anomalies.
- Type
- Brief Report
- Information
- Copyright
- © 2005 Cambridge University Press
Interventions in children with congenital cardiac malformations are increasing in importance and possibilities. Initial attempts to close ventricular septal defects with devices made for other purposes1–3 sometimes had serious and unwanted results.4 Since Amplatz et al. launched the devices designed specifically first for muscular,5 and later for perimembranous,6 ventricular septal defects, there has been an increasing interest in this method. Recent reports of closure of muscular,7 and even perimembranous,8 defects show that this may be a good alternative. In our institution, we started to use the specialised Amplatzer™ devices for selected defects in 2002, both in the muscular as well as in the perimembranous variants. One of our patients with a perimembranous defect had a right-sided heart, with mirror-imaged arrangement of the organs, including the atrial appendages. We describe the treatment of this specific patient.
Case report
The patient was a boy of 6.3 years, 113 centimetres tall, and with a body weight of 15.9 kilograms. He had a history of multiple pulmonary infections, as well as sinusitis. Findings of ciliac dysfunction, along with the right-sided location of his heart, and the mirror-imaged arrangement of all organs, were indicative of the classical Kartagener syndrome.9 The ventricular septal defect was initially thought to be small, and of little importance, but he was referred to our hospital for review.
We found the typical clinical findings for a ventricular septal defect, with a harsh systolic murmur and a mid-diastolic rumble. The defect measured 5 millimetres on echocardiography, with the left-sided dimension being slightly increased. The electrocardiogram was typical for a left-sided morphologically right atrium and sinus node, and right-sided location of the ventricular mass. The chest X-ray confirmed that the heart shadow was predominantly to the right, and showed mirror-imaged arrangement of the lungs. The pulmonary vascular markings were a little increased. The findings matched our usual standards for closure. After thorough oral, as well as written, information the parents were given two alternatives. After having considered the options at home, they chose the interventional approach rather than surgery.
The catheter procedure
We used general anaesthesia, and approached the heart percutaneously both through the vein and artery in the right groin, performing the procedure under echocardiographic and fluoroscopic guidance. Oxymetry indicated increased pulmonary blood flow, just above 1.5 times the systemic blood flow, and the right ventricular pressure was normal. Left ventriculography and transoesophageal echocardiography led us to suggest that the defect was less than 5 millimetres in diameter. We crossed the defect using a cobra-shaped catheter from the left side. A wire was pushed through this retrograde catheter and into the pulmonary artery, snared with an Amplatz Goose-neck™ snare (Fig. 1a), and pulled out through the femoral vein, thus creating an arteriovenous wire loop. Over this wire, the delivery system was passed through the vein, and across the ventricular septal defect into the apex of the left ventricle in mirror-imaged fashion (Fig. 1b). A 6-millimetre Amplatzer™ perimembranous ventricular septal defect occluder was inserted through the sheath. This device, however, was very easily pulled through the defect. This led us to conclude that we had underestimated the size of the defect. We re-created the loop, again without difficulties, and implanted an 8-millimetre device with ease (Fig. 2a). The immediate angiographic picture demonstrated near-to-complete closure (Fig. 2b). The total procedure time was 130 minutes, with fluoroscopy lasting for 35 minutes.
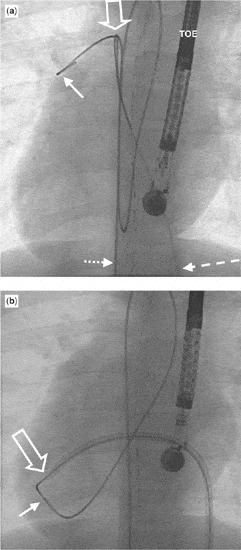
Figure 1. (a) The guide wire (dotted, short arrow) passed from the femoral artery, right-sided aorta and left ventricle across the ventricular septal defect and into the pulmonary trunk (solid arrow). It has been snared in the stem of the pulmonary trunk (open arrow), with a goose neck snare entered through the femoral vein (long dotted arrow). The echo probe (TOE) is in the oesophagus. As shown in (b), the loop runs from the femoral artery, through the right-sided aorta and the ventricular septal defect into the right ventricle, further into the inferior caval vein positioned on the left side, and out of the femoral vein. The delivery sheath has been inserted through the vein, right atrium and ventricle, through the ventricular septal defect and into the apex of the left ventricle (open arrow). The guide wire is shielded with a catheter, the solid arrow indicating its tip. The set-up is ready for implantation of the device.
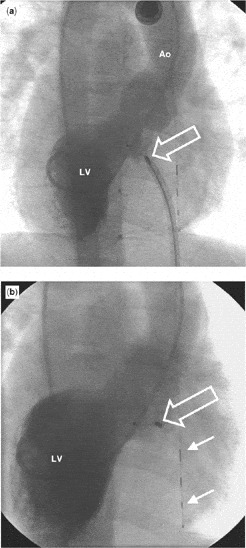
Figure 2. Angiocardiography (a) was performed in the cranially angled right anterior oblique view with injection of contrast medium into the left ventricle (LV) and filling of the aorta (Ao) as a last check before detaching the device. The delivery cable is still attached to the connection screw of the device (open arrow). The position of the device seems to be correct, and there is at most a minimal leakage of contrast medium into the right ventricle. The final angiogram (b), taken in the same projection as in (a) with injection of contrast medium into the left ventricle (LV) after release of the device, demonstrates its good position (open arrow). The shunt is no longer present. The guide wire in the oesophagus is used for calibration of measurements (two solid arrows).
The patient was discharged the following day after clinical and echocardiographic studies had shown that the defect now was completely closed. The patient has now been followed for one year. As far as the pulmonary problems are concerned, the clinical condition has improved. He has an unchanged normal rhythm in his electrocardiogram and there have been no unfavourable incidents.
Discussion
After catheter-based treatment for perimembranous septal defects became realistic with the launch of the Amplatzer™ devices,6 the technique has gained increasing interest among paediatric cardiologists, with experience suggesting it to be a good therapeutic option.8, 10 Our preliminary experience supports this view, and the procedure was uneventful even in our patient with a right-sided heart. We conclude, therefore, that catheter-based closure of perimembranous septal defects may be performed with optimal results, even when the heart is abnormally located.
References

(a) The guide wire (dotted, short arrow) passed from the femoral artery, right-sided aorta and left ventricle across the ventricular septal defect and into the pulmonary trunk (solid arrow). It has been snared in the stem of the pulmonary trunk (open arrow), with a goose neck snare entered through the femoral vein (long dotted arrow). The echo probe (TOE) is in the oesophagus. As shown in (b), the loop runs from the femoral artery, through the right-sided aorta and the ventricular septal defect into the right ventricle, further into the inferior caval vein positioned on the left side, and out of the femoral vein. The delivery sheath has been inserted through the vein, right atrium and ventricle, through the ventricular septal defect and into the apex of the left ventricle (open arrow). The guide wire is shielded with a catheter, the solid arrow indicating its tip. The set-up is ready for implantation of the device.

Angiocardiography (a) was performed in the cranially angled right anterior oblique view with injection of contrast medium into the left ventricle (LV) and filling of the aorta (Ao) as a last check before detaching the device. The delivery cable is still attached to the connection screw of the device (open arrow). The position of the device seems to be correct, and there is at most a minimal leakage of contrast medium into the right ventricle. The final angiogram (b), taken in the same projection as in (a) with injection of contrast medium into the left ventricle (LV) after release of the device, demonstrates its good position (open arrow). The shunt is no longer present. The guide wire in the oesophagus is used for calibration of measurements (two solid arrows).
- 2
- Cited by


