Foetal or intrauterine growth restriction affects between 5 and 10% of pregnancies and is associated with a number of neonatal complications, neonatal mortality, and long-term morbidities.Reference Wang, Fu and Liu 1 Intrauterine growth restriction can be further divided into two subgroups: early-onset intrauterine growth restriction with abnormal umbilical artery Doppler velocity, and late-onset intrauterine growth restriction or small-for-gestational-age foetuses with normal umbilical artery Doppler findings. Intrauterine growth restriction can cause multifactorial damage to foetuses and neonates; cardiac remodelling, among the short-term changes in the cardiovascular system, is part of the foetal pathophysiologic mechanism for adaptation to placental insufficiency and hypoxia.Reference Bozynski, Hanafy and Hernandez 2 , Reference Makikallio, Vuolteenaho, Jouppila and Rasanen 3 While cardiac remodelling and combined systolic and diastolic dysfunction have always been associated with the most severely affected intrauterine growth restriction foetuses,Reference Crispi, Hernandez-Andrade and Pelsers 4 more recent studies have shown that even late-onset intrauterine growth restriction or small-for-gestational-age foetuses have signs of cardiac dysfunction.Reference Comas, Crispi, Cruz-Martinez, Figueras and Gratacos 5 This cardiovascular adaptation in utero persists later in childhood in the form of subclinical cardiac dysfunctionReference Crispi, Figueras, Cruz-Lemini, Bartrons, Bijnens and Gratacos 6 , Reference Crispi, Bijnens and Figueras 7 and can also be associated with an increase in cardiovascular mortality in adulthood.Reference Barker, Osmond, Golding, Kuh and Wadsworth 8 , Reference Kaijser, Bonamy and Akre 9
Although the long-term effects of cardiovascular foetal remodelling have been assessed in children with intrauterine growth restriction, few studies have reported its consequences at birth.Reference Leipälä, Boldt, Turpeinen, Vuolteenaho and Fellman 10 – Reference Sehgal, Doctor and Menahem 12 Therefore, the aim of the study was to assess cardiac morphometry, systolic, and diastolic function in newborns with late-onset intrauterine growth restriction during the early haemodynamic adaptation to extrauterine life to test the hypothesis that intrauterine growth restriction affects cardiac shape and causes functional changes which can lead to systolic or diastolic dysfunction at birth.
Materials and methods
Patients
A prospective cohort study of 50 newborns delivered after 34 weeks of gestational age was conducted. In all pregnancies, gestational age was calculated based on the crown–rump length at first-trimester ultrasound.Reference Robinson, Sweet and Adam 13 All patients were assessed prenatally and postnatally in the maternity or neonatal unit in Sant Joan de Déu Hospital. Patients were divided into two groups according to the birth weight centile using local reference curves. Those with a birth weight centile equal to or lower than 10 were classified as intrauterine growth restriction. The control group was formed by newborns with a normal weight and height at birth with no pregnancy complications. Exclusion criteria were structural or chromosomal anomalies, twin pregnancies, CHD, or pulmonary hypertension. Neonates with a birth weight centile >90, those born by in vitro fertilisation, or from mothers who smoked were also excluded. Maternal characteristics and perinatal data were obtained from medical records and by interviewing parents at enrolment.
Foetal ultrasound
Cases and controls were studied in foetal life following a standardised protocol, using a Siemens Sonoline Antares ultrasound system (Siemens Medical Systems, Malvern, PA, United States of America). Foetoplacental Doppler assessment included uterine artery, umbilical artery, middle cerebral artery, and ductus venosus pulsatility indexes. Cerebroplacental ratio was defined as the ratio of the middle cerebral artery pulsatility index divided by the umbilical artery pulsatility index.Reference Baschat and Gembruch 14
Perinatal data
Pregnancy and neonatal outcome were assessed. Gestational age at delivery and mode of delivery were recorded. Neonatal anthropometric data, including weight, height, and body surface area were measured on admission. Percentiles were calculated using local reference curves. Neonatal outcome included Apgar score, admission to the neonatal ICU, neonatal death, bronchopulmonary dysplasia, neonatal respiratory distress syndrome, grade III/IV intraventricular haemorrhage, necrotising enterocolitis, and sepsis.
Echocardiography
Newborns were studied when resting quietly or asleep by two experienced physicians (L.R., J.B.). Cardiac scans were performed with a Philips iE33 (iE33, Philips Ultrasound, Bothell, WA, United States of America) Echocardiography System with a S8-3 MHz sector array following a standardised protocol. A comprehensive examination to rule out structural heart anomalies or pulmonary hypertension was carried out prior to the study. Cardiac morphometry, systolic, and diastolic function were assessed by two-dimensional, pulsed Doppler, and tissue Doppler imaging. The images were recorded and stored. They were analysed later, blinded to the group of the patient. All the measurements were averaged over three consecutive beats to account for respiratory variation.Reference Lopez, Colan and Frommelt 15
Cardiac morphology
Left and right sphericity indexes were calculated as base-to-apex length divided by basal diameter, measured in an end-diastolic two-dimensional apical four-chamber view, at the frame with the maximum chamber intraluminal area. Left ventricular septal and posterior wall thickness and chamber dimensions in systole and diastole were measured by using M-mode in a parasternal long-axis view. Relative wall thickness was calculated as the sum of the posterior wall and interventricular septum thickness divided by the left ventricular diastolic diameter.Reference Lopez, Colan and Frommelt 15 , Reference Lowes, Gill and Abraham 16
Systolic function
Systolic function assessment included heart rate, left and right stroke volume, left and right cardiac output, left shortening fraction by M-mode, mitral and tricuspid annular displacement (mitral/tricuspid annular plane systolic excursion), and systolic annular peak velocity (s′) using tissue Doppler imaging. The calculations of stroke volume and cardiac output were performed using the following equations: Stroke volume=(Cross-sectional aortic or pulmonary annulus area)2×0.785×Aortic or Pulmonary flow velocity−time integral. Cardiac output=Stroke volume×Heart rate.Reference Connolly and Oh 17
Aortic annulus was measured with magnification in a parasternal long-axis view, and pulmonary annulus in a parasternal short-axis view, from one inner edge to the opposite inner edge at the moment of maximum expansion in systole.Reference Lopez, Colan and Frommelt 15 The aortic flow velocity–time integral (VTI) was measured at the level of the aortic valve in an apical five-chamber view and the pulmonary velocity–time integral at the level of the pulmonary valve in a parasternal short-axis view.Reference Connolly and Oh 17
Systolic mitral and tricuspid motion were assessed by M-mode at the lateral mitral annulus (mitral annular plane systolic excursion) and the lateral tricuspid annulus (tricuspid annular plane systolic excursion) and by spectral tissue Doppler (s′) at the lateral and septal mitral annulus and the lateral tricuspid annulus in an apical four-chamber view.Reference Lopez, Colan and Frommelt 15 The measurements were made with the maximum possible alignment between the line of interrogation and the direction of annular motion. A sample volume gate length of 2.5 mm and sweep speed of 100 mm/second were used with simultaneous electrocardiographic display.
Diastolic function
Diastolic function was evaluated by early (e′) and late (a′) diastolic annular peak velocities at the medial and lateral mitral and lateral tricuspid annulus using tissue Doppler in an apical four-chamber view. Early and late transvalvular filling ratio and E deceleration time were assessed by pulsed Doppler from mitral and tricuspid inflow velocities.Reference Lopez, Colan and Frommelt 15
Cardiac time intervals
Isovolumetric times were measured from the spectral tissue Doppler waveform of the tricuspid, lateral, and septal mitral annular motion. They included isovolumetric contraction time′ and isovolumetric relaxation time′. Isovolumetric contraction time′ was measured from the end of the a′ wave to the onset of the s′ wave and isovolumetric relaxation time′ from the end of the s′ wave to the onset of the e′ wave.Reference Lopez, Colan and Frommelt 15 The ejection time′ was also measured in the spectral tissue Doppler waveform and the calculation of the myocardial performance index or Tei index was performed as follows: Tei index=(Isovolumetric relaxation time′ + Isovolumetric contraction time′)/Ejection time′.Reference Cui, Roberson, Chen, Madronero and Cuneo 18
The isovolumetric relaxation time′ and isovolumetric contraction time′ were also corrected for the heart rate by dividing isovolumetric relaxation time′ and isovolumetric contraction time′ by the square root of the R–R interval to eliminate the effect of heart rate on the isovolumetric times.Reference Cui, Roberson, Chen, Madronero and Cuneo 18
Statistics
The data were analysed with SPSS Statistics 19.0 software (IBM). The study outcome was cardiovascular assessment at birth. The independent variable of interest was the history of intrauterine growth restriction or normal foetal growth based on the weight at birth. Results are presented as mean±standard deviation or percentage as appropriate. Comparisons between groups were performed by using the χ2 test and linear regression. Echocardiographic parameters are shown as crude values and adjusted for body surface area at birth by linear regression analysis. A p-value <0.05 was considered statistically significant.
Results
Perinatal data, foetal ultrasound, and clinical outcome
Perinatal data, foetal ultrasound findings, and outcome are shown in Table 1. Maternal characteristics showed no differences between the two groups. All cases had normal umbilical artery pulsatility index as part of the definition of late-onset intrauterine growth restriction. Nevertheless, foetuses with intrauterine growth restriction had worse prenatal Doppler ultrasound findings. The uterine artery pulsatility index in foetuses with intrauterine growth restriction was significantly higher (p=0.006) and the cerebroplacental ratio significantly lower (p=0.032). Regarding perinatal outcome, even though gestational age at delivery was significantly lower in neonates with intrauterine growth restriction (p=0.001), there were no differences in the Apgar scores. Although the admission to a neonatal ICU was higher among neonates with intrauterine growth restriction (p⩽0.001), there were no cases of neonatal death or neonates with comorbidities such as bronchopulmonary dysplasia, grade III/IV intraventricular haemorrhage, necrotising enterocolitis, or sepsis in the study groups. The reasons for admission to the neonatal ICU among neonates with intrauterine growth restriction were as follows: low birth weight n=7, transient tachypnea of the newborn n=2, hypoglycaemia n=2, and one infant who developed a mild form of neonatal respiratory distress syndrome soon after admission to the maternity ward. Only one control was admitted with transient tachypnea of the newborn.
Table 1 Maternal and foetal characteristics.

IUGR=intrauterine growth restriction; PI=Pulsatility index
Data are presented as mean±SD or (%)
Neonatal echocardiographic assessment
Results for cardiac morphology and function are shown in Table 2 and Fig 1. Cardiac shape showed significant differences between the study groups. The left and right ventricular sphericity indexes were lower in the intrauterine growth restriction group (p crude⩽0.001) due to longer left and right basal diameters (p=0.027 and 0.019, respectively) when adjusted for body surface area.
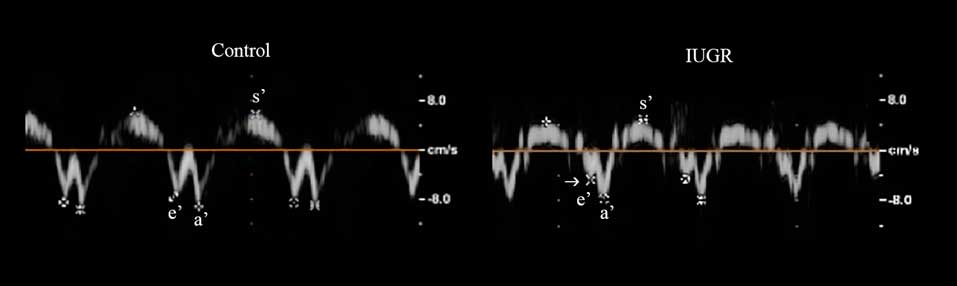
Figure 1 Echocardiographic images in a control (left) and in a neonate with intrauterine growth restriction (IUGR) (right). Spectral tissue Doppler at the septal mitral annulus, illustrating the lower early diastolic (e′) myocardial peak velocity in the IUGR (arrow).
Table 2 Neonatal cardiac assessment.

E/A=early and late transvalvular filling ratio; ET=ejection time; IUGR=intrauterine growth restriction; IVCT=isovolumetric contraction time; IVRT=isovolumetric relaxation time; MAPSE=mitral annular plane systolic excursion; TAPSE=tricuspid annular plane systolic excursion
Data are presented as mean±SD
* P1=crude
** P2=adjusted for body surface area
Although heart rate and left shortening fraction were similar between groups, cases showed higher left stroke volume (p=0.009). The mitral annular plane systolic excursion, lateral, and septal mitral annulus s′ were smaller in the intrauterine growth restriction group, but these differences disappeared when the results were adjusted for body surface area. Regarding right systolic function, the right ejection volume and cardiac output were similar between groups. Although the tricuspid annular plane systolic excursion was significantly lower in the intrauterine growth restriction neonates (p=0.050), the tricuspid annulus s′ in the tissue Doppler showed no differences.
Diastolic function in both left and right ventricles showed similar results except for septal mitral annulus e′, which was lower in the intrauterine growth restriction group (p=0.021).
When looking at the isovolumetric times and myocardial performance index (Tei index), the most striking differences were seen in the right heart. The tricuspid annulus “isovolumetric contraction time′” and “isovolumetric relaxation time′” were significantly longer (p crude=0.016 and 0.032, respectively), and at the same time, the “ejection time′” showed a tendency to be shorter in intrauterine growth restriction newborns (p crude=0.076). Therefore, the tricuspid annulus Tei index was significantly higher in the intrauterine growth restriction group than in the control group (p crude=0.002). When correcting the isovolumetric times for the heart rate, the differences were even more pronounced between the groups (tricuspid annulus-corrected “isovolumetric contraction time′” p crude=0.009 and corrected “isovolumetric relaxation time′” p crude=0.011).
Overall, there was a significant tendency to worse cardiac function results in the right heart in intrauterine growth restriction neonates as compared to controls.
Discussion
The cardiac remodelling shown in intrauterine growth restriction foetuses and newborns is not associated exclusively with the most severe cases of intrauterine growth restriction, and this study provides echocardiographic evidence that cardiac morphology and function can be affected even in small-for-gestational-age neonates at birth. The study group was formed by neonates with late-onset intrauterine growth restriction with significant changes in the uterine artery Doppler and cerebroplacental ratio in the prenatal ultrasound, which showed that placental insufficiency and haemodynamic redistribution took place in the foetal life.Reference Turan, Turan and Gungor 19
One of the most striking echocardiographic features in intrauterine growth restriction neonates was the change in the shape of the heart. Neonates with intrauterine growth restriction showed more globular hearts, mainly due to a relative increase in the basal diameter, more pronounced in the right heart. These changes in cardiac shape have been previously described in older children and could be the result of the combined effects of increased pressure overload due to elevated placental vascular resistance and chronic myocardial hypoxia.Reference Crispi, Figueras, Cruz-Lemini, Bartrons, Bijnens and Gratacos 6 , Reference Crispi, Bijnens and Figueras 7 A previous study in neonates has also found left atrial dilatation in neonates with history of intrauterine growth restriction.Reference Sehgal, Doctor and Menahem 12 Even though previous studies in foetuses and neonates with intrauterine growth restriction have shown cardiac hypertrophy,Reference Leipälä, Boldt, Turpeinen, Vuolteenaho and Fellman 10 , Reference Veille, Hanson, Sivakoff, Hoen and Ben-Ami 20 the cardiac wall thickness was similar between the groups. In concordance with the data documented by Crispi et al, cardiac hypertrophy was not present in late-onset intrauterine growth restriction neonates.Reference Crispi, Figueras, Cruz-Lemini, Bartrons, Bijnens and Gratacos 6 , Reference Crispi, Bijnens and Figueras 7
When assessing the systolic function, there was no difference in heart rate, but left stroke volume was higher in the intrauterine growth restriction group. Previous studies in neonatesReference Sehgal, Doctor and Menahem 12 and childrenReference Crispi, Bijnens and Figueras 7 have found a reduced left stroke volume in cases with intrauterine growth restriction. This could be a result of the severity of intrauterine growth restriction, as explained by Crispi et al; the reduced systolic volume was only seen in children with a history of severe intrauterine growth restriction and not in those with mild forms.Reference Crispi, Bijnens and Figueras 7 In a neonatal study,Reference Sehgal, Doctor and Menahem 12 the selection of cases included neonates with intrauterine growth restriction with a birth weight centile <3, whereas in our study the cases included had a birth weight centile ⩽10. The increase in stroke volume seen in the current study has also been previously documented in neonates with intrauterine growth restriction at birthReference Leipälä, Boldt, Turpeinen, Vuolteenaho and Fellman 10 , Reference Martinussen, Brubakk, Vik and Yao 21 and it has been hypothesised that it acts as a compensatory mechanism for foetal tissue hypoxia. As the mitral annular plane systolic excursion remained similar, the higher stroke volume could be the result of an increased radial contractility, supported by a higher left ventricular wall thickening in intrauterine growth restriction children.Reference Crispi, Figueras, Cruz-Lemini, Bartrons, Bijnens and Gratacos 6 The higher stroke volume could also be explained as the result of a greater radial diameter. In globular hearts, the increase in the radial diameter is a mechanism that leads to greater stroke volume with a similar shortening fraction.Reference Marciniak, Claus and Sutherland 22 Therefore, the cardiac output could be achieved without a further increase in the heart rate, as observed in this study. The tricuspid annular plane systolic excursion was the only right systolic value which showed differences between the groups when adjusted to body surface area. A decrease in the tricuspid annular plane systolic excursion has been documented in children with a history of intrauterine growth restriction,Reference Crispi, Figueras, Cruz-Lemini, Bartrons, Bijnens and Gratacos 6 , Reference Crispi, Bijnens and Figueras 7 but can only be a marker of right systolic dysfunction once pulmonary hypertension has been ruled out.Reference Grangl, Pansy, Burmas and Koestenberger 23
Even though lateral tricuspid and septal early diastolic (e′) peak myocardial velocities were smaller in intrauterine growth restriction neonates, these only remained statistically significant at the septal annulus when adjusted for body surface area. Subclinical diastolic dysfunction could be the consequence of intrauterine cardiac remodelling.Reference Crispi, Bijnens and Figueras 7 It has been documented in both small-for-gestational-ageReference Comas, Crispi, Cruz-Martinez, Figueras and Gratacos 5 and severe cases of intrauterine growth restriction foetusesReference Cruz-Lemini, Crispi and Valenzuela-Alcaraz 24 and neonates;Reference Sehgal, Doctor and Menahem 12 moreover, it can persist in later childhood.Reference Crispi, Bijnens and Figueras 7
Cardiac time intervals – isovolumetric contraction time′ and isovolumetric relaxation time′ – were also corrected for heart rate (corrected isovolumetric contraction time′ and corrected isovolumetric relaxation time′). As isovolumetric contraction time′ and isovolumetric relaxation time′ decrease linearly with heart rate, heart rate correction eliminates the effect of the heart rate on the isovolumetric times.Reference Cui, Roberson, Chen, Madronero and Cuneo 18 As body surface area does not correlate with time intervals,Reference Cui, Roberson, Chen, Madronero and Cuneo 18 the most striking results were found at the lateral tricuspid annulus as both isovolumetric contraction time′ and isovolumetric relaxation time′ crude values were longer in the intrauterine growth restriction neonates, and these differences increased further after correction for heart rate. A prolonged left isovolumetric relaxation time measured by pulsed wave Doppler was also previously described in neonates with intrauterine growth restriction.Reference Sehgal, Doctor and Menahem 12
The myocardial performance index or Tei index is a known marker of combined systolic and diastolic function.Reference Tei, Ling and Hodge 25 In systolic dysfunction, the isovolumetric contraction time increases and, similarly, a rise in the isovolumetric relaxation time is seen in diastolic dysfunction. Therefore, an increase in the index can be a sign of systolic, diastolic, or combined cardiac dysfunction, and appears to be relatively independent of preload or afterload, but load dependence of the Tei index needs to be further elucidated.Reference Tei, Nishimura, Seward and Tajik 26 , Reference Eidem, O’Leary, Tei and Seward 27 In the present study, a significant difference in the Tei index was only found in the right heart in the crude value. As the tissue Doppler-derived Tei index at the tricuspid annulus is independent of body surface area according to Cui et al,Reference Cui, Roberson, Chen, Madronero and Cuneo 18 the results found support the existence of combined systolic and diastolic dysfunction in the right heart at birth in neonates with intrauterine growth restriction.
Finally, when looking at the short-term outcome of these newborns, admission to the neonatal unit is more common, which is to be expected due to the lower birth weight of the newborns with intrauterine growth restriction, but there was no difference in major morbidities and mortality, and there was no need for neonatal resuscitation at birth.
The main limitations of the present study are the small number of patients in each group and the variability that could arise in terms of the variation in pulmonary vascular resistance in each patient. In order to reduce the influence of the increased pulmonary pressures on the right heart, pulmonary hypertension was excluded. Furthermore, the investigators were not blinded to the echocardiographic study, but the measurements were done without any data regarding patient anthropometric or personal information.
In conclusion, neonates with late-onset intrauterine growth restriction showed cardiac remodelling and signs of systolic and diastolic dysfunction at birth. The adaptation to extrauterine life occurred with more globular hearts, higher stroke volumes but a similar heart rate compared to adequate-for-gestational-age neonates. These differences appear more significant in the right heart, in concordance with its major function in foetal life. Since intrauterine growth restriction is a well-known risk factor for cardiovascular disease, this paediatric population could benefit from long-term follow-up as well as prompt adoption of preventive measures of cardiovascular disease.
Acknowledgements
The authors acknowledge the work done by the research team based in Barcelona Center for Maternal Fetal and Neonatal Medicine.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and have been approved by Sant Joan de Déu Hospital ethical committee. Informed parental consent was obtained before inclusion.






