The circuit of cardiopulmonary bypass must be filled with fluid solution in order to start extracorporeal circulation with adequate flow rates and no risk of air embolism. In the past, homologous blood was usually added to the priming volume to limit haemodilution. It was apprehended that reduced oxygenation capacity following decreased haemoglobin concentration after inducing extracorporeal circulation would damage vital organs, brain and kidneys in particular. It was also presumed that dilutional coagulopathy could ensue. On the one hand, an optimal value of lowest haematocrit during open-heart surgery has not yet been established. Moreover, haemodilution can improve tissue perfusion and oxygen delivery by decreasing blood viscosity and releasing peripheral vascular resistance.Reference Cooper and Giesecke 1 On the other hand, the use of stored homologous blood carries a number of risks such as disease transmission, anaphylactic reaction, and immunologic sensitisation. It can also contribute to coagulation dysfunction. In light of these considerations, even though, according to Patel et al,Reference Patel, Avlonitis, Jones, Reeves, Sterne and Murphy 2 there is no evidence from randomised studies that liberal thresholds for red blood cell transfusion are associated with a substantially increased risk of mortality and morbidity, homologous blood should ideally not be used for the priming solution of cardiopulmonary bypass circuit. This recommendation can easily be followed in adult patients whose circulatory volume is large enough to prevent excessive haemodulition. It necessarily implies drastic volume reduction of the entire bypass circuit in the young paediatric population.
We currently perform all open-heart procedures using priming solutions without donor blood for all patients, regardless of their age and their weight. A recent article from our institution showed that shorter duration of extracorporeal circulation and high preoperative haemoglobin concentration supported transfusion-free operation in neonates. On the contrary, this was rarely achieved during univentricular palliative procedures such as the Norwood procedure.Reference Boettcher, Sinzobahamvya and Miera 3 However, that study used a univariate analysis and the number of tested variables was limited. This article performs multivariate analysis including several variables pertaining to successful achievement of complete transfusion-free operation in a greater number of neonates and infants of a low body weight.
Patients and methods
Data for 498 consecutive patients, younger than 366 days and with a body weight not more than 7 kg, who underwent open-heart surgery from January, 2014 to December, 2016 were reviewed.
The study included 158 neonates. Body weight at the time of surgery ranged from 1.48 to 7.0 kg with a mean of 4.56±1.31 kg. It was less than 2.5 kg in 15 instances and comprised between 2.5 and 3.5 kg in 123 patients. Patients’ main characteristics did not vary during the 3-year period of this study. In particular, the complexity of procedures carried out according to the Aristotle basic complexity scoreReference Lacour-Gayet, Clarke and Jacobs 4 (8.7±2.4), their mortality score (1.12±1.01), and their difficulty ranking (84±39) in conformity with categories of the Society of Thoracic Surgery and the European Association of Cardiothoracic SurgeryReference O’Brien, Clarke and Jacobs 5 were similar.
Blood-sparing approach
A comprehensive blood-sparing approach was routinely followed. For elective surgery, when haemoglobin concentration was below normal range, iron supplementation was provided. Unnecessary blood sampling before operation was avoided. No blood or blood product was added to the priming solution of the cardiopulmonary circuit. The bypass circuit was adapted to patient’s weight so that the predicted haemoglobin concentration, calculated from the haemoglobin concentration before bypass onset, priming volume, and estimated blood volume,Reference Redlin, Boettcher, Dehmel, Cho, Kukucka and Habazettl 6 was higher than the chosen threshold (minimal value) to indicate blood transfusion during extracorporeal circulation. This has been fixed to 8.0 g/dl during cardiopulmonary bypass course and 13 g/dl after coming off bypass in cases of palliative surgery with maintained cyanosis as recently reported.Reference Boettcher, Sinzobahamvya and Miera 3 Efforts were made to avoid transfusion during extracorporeal circulation or, at least, to postpone transfusion towards its end. The surgeon paid special attention to operative technique – chest entry, dissection, cannulation – in order to minimise blood loss. Conventional ultrafiltration limited haemodilution, when required. After coming off bypass, the residual blood in the pump circuit was drawn into syringes and was directly given back to the patient. The surgeon, anaesthetist, and perfusionist regularly consulted with each other to coordinate this blood-sparing strategy.
Adjustment of cardiopulmonary bypass circuit
The adjustment of cardiopulmonary circuit has already been described.Reference Boettcher, Sinzobahamvya and Miera 3 The kind and size of the oxygenator, tubing, and arterial pump boot that were used according to patient’s body weight are detailed in Table 1. Mast-mounted pumps were adjusted in height and position to be as close as possible to the operation table, with the venous reservoir at the same height as the patient’s right atrium, in order to minimise tubing length. We could thus reduce the priming volume to 73 ml for the smallest patients with body weight up to 2.5 kg, and to 110 ml for those with a body weight between 5.1 and 7.0 kg.
Table 1 Weight-adjusted cardiopulmonary bypass circuits.
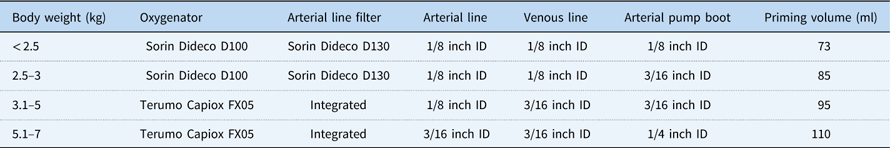
ID=inner diameter
Surgical management
The newborns and infants were usually pre-medicated by midazolam (0.1 mg/kg), and induction of anaesthesia was in most cases started with sufentanil (1 µg/kg) or etomidate (0.3 mg/kg), the latter mainly in patients with limited haemodynamic reserve. Rocuronium (1 mg/kg) was used as an intermediate-acting steroidal non-depolarising neuromuscular blocker. Maintenance of anaesthesia was assured by remifentanil (0.4–0.7 µg/kg/hour) and propofol at a dose of 5 mg/kg/hour. Perioperative monitoring included estimation of cerebral and peripheral oxygenation by near-infrared spectroscopy and regular measurement of plasma lactate level in order to keep it lower than 2 mmol/L as long as possible. Any major decline of regional oxygen saturation was responded to, first, by controlling the correct position of venous and arterial cannulas and, second, by augmenting the extracorporeal circulation flow rate and, only if these interventions were insufficient, by adding donor blood to the bypass circuit.
All operations were performed through a median sternotomy. It was a second chest re-entry on 88 occasions. Operation was conducted in normothermia in 49 patients. Otherwise, hypothermia ranged from 24° to 35°C, with a mean of 30±2.5°C and a median of 32°C. To avoid complete circulatory arrest, procedures involving aortic arch reconstruction were performed during episodes of cerebral perfusion through innominate artery and distal aortic perfusion either via descending thoracic aorta or common femoral artery. Table 2 details the main operations that were carried out at least eight times. The three most frequent among these were patch repair of ventricular septal defect 58 times, repair of tetralogy of Fallot on 46 occasions, and arterial switch operation in 44 cases. Mean duration of extracorporeal circulation and reperfusion time was 176±48 minutes. Procedures were performed under beating hearts in 78 instances and mean aortic cross-clamping time was 89±39 minutes in the other cases.
Table 2 Most frequently performed procedures: rate in % of successful transfusion-free operation and early postoperative mortality.

AVSD=atrioventricular septal defect; DORV=double-outlet right ventricle; PA=pulmonary artery; RVOT=right ventricular outflow tract; TAPVC=totally anomalous pulmonary venous connection; TOF=tetralogy of Fallot; VSD=ventricular septal defect
* Three deaths after shunt, systemic to pulmonary, central, and one death after implantation of left ventricular assist device for acute myocarditis, aortopulmonary window repair, Damus–Kay–Stansel procedure, Rastelli operation, and Ross–Konno procedure, respectively
Statistical analysis
The general characteristics of the 3-year study period were compared using one-way analysis of variance. In total, 27 factors that could influence the achievement or the non-achievement of complete transfusion-free procedure were first tested individually using an unpaired t-test, Mann–Whitney test, and χ2 test, as indicated. Variables with a p-value lower than 0.2 were retained for further multivariate analysis by logistic regression using XLSTAT software. Statistical significance was determined as a p-value lower than 0.05. Graphpad Prism (San Diego, California, United States of America) computed the best non-linear regression curves. Means are reported with standard deviation, and percentages with 95% confidence interval when appropriate.
Written consent was obtained from all parents or guardians to use anonymised data for research, teaching, and external quality control.
Results
Cardiopulmonary bypass could be conducted without any transfusion of blood or blood products in 335 procedures (67% of cases) (Fig 1). Transfusion-free operation was achieved in 136 patients (27% of cases) and was more frequently observed, as noted in Table 2, after arterial switch operation associated with repair of ventricular septal defect (66.7%). It was never observed after a Norwood procedure (0%).

Figure 1 Application of blood transfusion during operations with the use of cardiopulmonary bypass (CPB).
Perioperative transfusion was needed in 362 patients, because of a haemoglobin concentration that was too low on 186 instances, insufficient oxygenation (cyanosis) on 95 occasions, hypovolaemia in 35 patients, coagulation disturbances and bleeding in 29 cases, and mixed reasons in 17 procedures. It was mostly carried out at the end of cardiopulmonary bypass (70 cases) or after coming off bypass (199 cases). With these 269 (70+199) cases, added to the 136 transfusion-free patients, our transfusion strategy was overall fulfilled on 405 occasions: that is, 81.3% (405/498) of procedures in this series.
Mean haemoglobin concentration decreased to 10.1±1.9 g/dl 30 minutes after starting extracorporeal circulation for patients who were not transfused during operation, and to 9.2±1.7 g/dl for those who were transfused. Haemoglobin levels stabilised thereafter during cardiopulmonary bypass, to finally increase at bypass end and chest closure, up to 11.1±2.6 g/dl and 13.6±2.6 g/dl, respectively, as shown in Figure 2.

Figure 2 Course of haemoglobin (Hb) concentration during operations with the use of cardiopulmonary bypass (CPB) for transfused (n=362) and not transfused (n=136) patients. The dashed lines indicate the standard deviation.
Factors associated with transfusion-free operation
With p-values not higher than 0.001 on univariate analysis, transfusion-free operation was significantly more often achieved by a certain team of surgeons and anaesthetists. It was supported by higher preoperative haematocrit and haemoglobin concentration, higher haemoglobin level after starting extracorporeal circulation, and shorter duration of cardiopulmonary bypass. It was less frequently achieved in the case of chest re-entry and in procedures with elevated mortality score.
Overall, 18 variables yielded p-values lower than 0.20 (Table 3). Logistic regression was applied and five factors were found to independently facilitate transfusion-free operation: lower mortality score (p=0.001), anaesthesia provided by a certain physician (p=0.006), first chest entry (p=0.013), higher haemoglobin concentration, before going on cardiopulmonary bypass (p=0.013), and after starting bypass (p=0.030).
Table 3 Factors associated with achievement of transfusion-free operation: univariate analysis and logistic regression.

ABC=Aristotle basic complexity; aPTT=activated partial thromboplastin time; CPB=cardiopulmonary bypass; Hb=haemoglobin; INR=International normalised ratio
Bold values indicate variables whose p-values are statistically significant
The 42 patients who underwent a procedure included in Society of Thoracic Surgery and European Association of Cardiothoracic Surgery mortality category 5, with mortality scores ranging from 2.7 to 5.0, that is 33 Norwood procedure, eight Damus–Kaye–Stensel procedure, and one double-switch repair of congenital corrected transposition of the great arteries, were all transfused during operation. Nevertheless, in 52% (22/42) of cases transfusion took place right before coming off bypass. The best computed non-linear regression curve concerning factor “mortality score” looks like a graph generated by a log(inhibitor) versus response equation (Fig 3).

Figure 3 Rate in % of transfusion-free operations according to procedures mortality score according to the Society of Thoracic Surgery and the European Association of Cardiothoracic Surgery.Reference Lacour-Gayet, Clarke and Jacobs 4 The best non-linear regression graph looks like generated by log(inhibitor) versus response equation: Y=Bottom + (Top-Bottom)/(1+10^ (X-LogIC50)). Bottom= −1.039, Top= 31.72, LogIC50=2.491. Goodness of Fit, r 2 is equal to 0.79. The rate is null for a mortality score equal to or higher than 3.78 (SD=0.44).
The rate at which “bloodless” procedure was achieved according to haemoglobin concentration after starting extracorporeal circulation is depicted in Figure 4, as a second-order polynomial formula. It increases to up to 40% for haemoglobin levels comprised between 12 g/dl and 14 g/dl and decreases slightly thereafter.

Figure 4 Rate in % of transfusion-free operations according to haemoglobin (Hb) concentration 5 minutes after starting cardiopulmonary bypass (CPB). The best computed non-linear regression graph follows second-order polynomial equation. The formula and the goodness of fit are displayed on the graph. Rate slightly decreases for Hb levels equal to or higher than 13.92 g/dl (SD=1.55 g/dl), most probably because of procedures dealing with cyanotic lesions with high Society of Thoracic Surgery and European Association of Cardiothoracic Surgery mortality score.Reference Lacour-Gayet, Clarke and Jacobs 4
With a p-value of 0.07, the variable “Cardiopulmonary bypass duration” almost attained statistical significance. Probability of successful transfusion-free procedure was as high as 85±2.7% at 60 minutes bypass interval, and as low as 13±3.6% at 270 minutes (Fig 5).

Figure 5 Kaplan–Meier curve depicting freedom from blood donor transfusion according to duration of cardiopulmonary bypass (CPB). The dashed lines delimit standard deviation.
Outcome
In total, 22 patients died in the hospital or in the first 30 postoperative days, for a global mortality of 4.4% (95% confidence interval: 2.8–6.6%). Details of involved procedures are given in Table 2. in total, 32 patients (95% confidence interval: 25–40 patients) were expected to die postoperatively according to the Society of Thoracic Surgery and the European Association of Cardiothoracic Surgery mortality score:Reference O’Brien, Clarke and Jacobs 5 an expected mortality of 6.4% (5.0–8.0%). With a p-value of 0.16, the difference between observed and expected mortality did not reach statistical significance.
Early postoperative mortality was much lower after transfusion-free operations than after procedures that required transfusion of blood or blood products: 0.7% (1/136) compared with 5.8% (21/362), p-value= 0.013. On the basis of univariate analysis, lower weight at time of surgery, higher mortality score, preoperative vasoactive-inotropic therapy, and longer duration of cardiopulmonary bypass were also found to be significant risk factors for early postoperative mortality (Table 4). Variables such as chest re-entry, different surgeons and anaesthetists, and haemoglobin concentration after starting extracorporeal circulation played no statistically significant role on survival. Only body weight (p=0.01) and duration of cardiopulmonary bypass (p=0.035) were found by logistic regression to be independent variables affecting early survival, as displayed in Table 4.
Table 4 Factors associated with early postoperative death.

CI=confidence interval; CPB=cardiopulmonary bypass
Bold values indicate variables whose p-values are statistically significant
There was no account of renal insufficiency requiring peritoneal dialysis, and no account of neurological deficit in this series. Transfused survivors needed longer mechanical ventilation (p=0.0002) and stayed longer in the ICU (p<0.0001). However, postoperative lactate level (p=0.21) and vasoactive-inotropic scoreReference Gaies, Jeffries and Niebler 7 , Reference Scherer, Moser, Brown, Rodefeld, Turrentine and Mastropietro 8 (p=0.47) were not statistically different between the two survivor groups. Median ICU stay was 5 days.
Of the 136 patients who were not transfused during operation, 19 (3.8%: 19/498 of total cases) could be discharged from hospital while not having had transfusion of blood or blood products during the entire hospital stay.
Discussion
The Society of Thoracic Surgeons blood conservation guidelines recommend minimising cardiopulmonary bypass circuit prime volume in adults as an integral blood conservation strategy. 9 In fact, open-heart surgery without homologous blood transfusion in adults is currently a generalised method, but not yet in children and infants. For such patients with small body weight, further reduction of bypass circuit volume is mandatory to contemplate “bloodless” cardiac surgery. Indeed, in most paediatric heart centres, lowest priming volumes are currently over 300 ml. Therefore, to prevent excessive haemodilution, homologous blood must be added in patients whose body weight is equal or less than 7 kg – that is neonates and young infants.
This study clearly demonstrates that, with the applied blood-saving strategy, it was feasible to conduct transfusion-free extracorporeal circulation in the majority (67%) of such paediatric population, regardless of weight or body surface area, and haemoglobin concentration could be maintained at an acceptable level, as shown in Figure 2. A significant number (27%) of patients did not require transfusion of blood or blood products during operation.
Our blood conserving strategy is not new. Several successful attempts to avoid transfusion in paediatric cardiac surgery by using miniaturised cardiopulmonary bypass circuits and stringent transfusion triggers have been reported, especially for children whose parents were Jehovah’s witnesses. However, our approach is novel as it is now applied routinely to all patients, regardless of the procedure, their age, and their weight. It is also noteworthy that we strive to postpone inevitable transfusion towards the last moments of extracorporeal circulation or after coming off bypass. This restricts transfusion through the arterial route, reducing the risk of haemolysis, as well as thrombo-embolism.Reference Ranucci, Carlucci and Isgro 10 , Reference Redlin, Boettcher, Kukucka, Kuppe and Habazettl 11
The greatest impediment to transfusion-free operation was a procedure mortality score higher than 2.6, that is Society of Thoracic Surgery and European Association of Cardiothoracic Surgery mortality category 5. In current surgical practice, this variable is not prone to modification. Similarly, the other robust adverse parameter “chest re-entry” cannot be changed. In contrast, the medical team involved should discuss measures to be implemented to correct the highly statistically significant effects of anaesthetists’ interventions. Finally, the fundamental role of haemoglobin concentration before cardiopulmonary bypass is validated. In case of anaemia, haemoglobin levels can be increased by supplementing iron. However, this measure obviously falls short in the face of rapid open-heart surgery requirements.
Contrary to previous publications,Reference Redlin, Habazettl and Boettcher 12 – Reference Miyaji, Kohira and Miyamoto 15 body weight at the time of operation was not a determinant. This ascertains the efficacy of our weight-adjusted miniaturisation of bypass circuit. By logistic regression, duration of cardiopulmonary bypass was not a predictor of intraoperative transfusion, although it may be taken into account additionally as p-value was low (p=0.07).
Lower body weight (p=0.01) and longer duration of cardiopulmonary bypass (p=0.035) appeared to be independent hazard factors for early postoperative mortality in this series, not perioperative transfusion (p=0.15).
The observed postoperative mortality for the whole cohort was relatively lower than expected. There was no account of renal insufficiency requiring peritoneal dialysis, and no account of neurological deficit. These facts plead for the safety of the applied cardiopulmonary bypass strategy.
Already in year 1999, Lau et alReference Lau, Posther and Stephenson 16 had noted that blood product transfusion enhances inflammatory response to extracorporeal circulation and increases myocardial and pulmonary dysfunction. Restricted use of homologous blood for extracorporeal circulation should be therefore promoted. This starts with asanguineous priming of cardiopulmonary bypass circuit. Even though most neonates and infants are transfused thereafter, this approach is worthwhile as the amount of transfused blood is overall reduced.Reference Redlin, Habazettl and Boettcher 12 Besides, it is associated with a lower postoperative mortality and morbidity.
Duration of mechanical ventilation and length of stay in ICU are the best-known surrogates for postoperative morbidity in congenital heart surgery.Reference Sinzobahamvya, Weber and Sata 17 This study establishes lower morbidity outcome for non-transfused survivors in conformity with a number of previous reports.Reference Boettcher, Sinzobahamvya and Miera 3 , Reference Redlin, Boettcher, Kukucka, Kuppe and Habazettl 11 , Reference Lau, Posther and Stephenson 16 , Reference de Gast-Bakker, de Wilde and Hazekamp 18 , Reference Kwak, Park, Lee and Lee 19 However, this was not the subject of this study. The analysis of postoperative morbidity was a univariate regression. A number of patients who were transfused during the operation suffered from lesions with higher mortality score. A multivariate analysis and propensity-score matching should be undertaken in order to address the issue.
Although data were prospectively collected, this is a retrospective study. Effects of asanguineous priming of CPB circuit on postoperative outcome could not be assessed by a control group, as all the patients who needed cardiopulmonary bypass in the study period were treated following this strategy. This somewhat constitutes a limitation of this study.
In conclusion, cardiopulmonary bypass could be conducted in the majority of cases without donor blood, regardless of body weight. Our blood-saving strategy was effectively achieved in 81.3% (405/498) of procedures. This resulted in a fair number of operations that were performed completely free of transfusion of blood or blood products. These were more often achieved in procedures with lower mortality score.
The surgical team has to collaborate to achieve transfusion-free open-heart surgery in neonates and infants. Indeed, as emphasised by Durandy,Reference Durandy 20 blood conservation is a perfect example of team work; everyone must be motivated to optimise reduction in blood use in the paediatric population.
Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflict of Interest
None.











