By 1938, Helen Taussig had directed the paediatric cardiology clinic at the Johns Hopkins Harriet Lane Home for nearly a decade. From clinicopathological correlations, Helen concluded that patients with tetralogy of Fallot were less cyanotic if they also had a persistent arterial duct, and that infants with tetralogy became more cyanotic when their arterial duct closed.
In 1939, surgeon Robert Gross, and paediatrician John Hubbard, working at Boston Children’s Hospital, were first to publish their success in surgically ligating the arterial duct, the procedure being performed by Gross in August of 1938.Reference Gross and Hubbard1 Unbeknownst to Hubbard or Gross, however, the surgeon Emil Karl Frey, working in Germany, had already ligated an arterial duct in early 1938, albeit that Frey did not report his experience until later.Reference Frey and Kuetgens2 The article published by Gross and HubbardReference Gross and Hubbard1 prompted Helen Taussig to expand her theory. She reasoned that, if surgeons could ligate an arterial duct, then maybe they could also create one. She concluded that, in patients with tetralogy of Fallot, surgical creation of an artificial arterial duct would improve their arterial oxygenation.
Background
Alfred BlalockReference Ravitch3–5
Alfred Blalock (Fig. 1) was born in Culloden, Georgia. He attended high school at the Georgia Military Academy. After graduation, he served in the Army during the First World War. Following the war, he received the degree of Bachelor of Arts from the University of Georgia, and subsequently attended The Johns Hopkins School of Medicine, graduating in 1922. While a medical student, and later as a resident, Blalock took part in research at the Hunterian animal research laboratory.

Figure 1 Alfred Blalock (1899–1964).
During his period at medical school, Blalock had hoped to be appointed to a general surgical internship at Johns Hopkins, but his grades were insufficient to achieve this position. Instead, he undertook an internship in urology at Hopkins, during which he distinguished himself. A few months into his urology internship, William Halstead, the Chief of Surgery, and a role model for Blalock, died. John M. T. Finney became acting chief. Finney was impressed by the performance of Blalock in urology, and he offered him a general surgery residency. Later, a dispute broke out between Blalock and the other residents in general surgery, leading Blalock to resign his position. It remains a fact that the principals failed then, or later, to reveal the exact reason why Blalock resigned from this residency. Despite this setback to his career, Blalock stayed at Hopkins until 1925, working as a resident in otolaryngology.
Blalock left Hopkins in the summer of 1925 with hopes of securing a general surgery residency at Peter Bent Brigham Hospital in Boston. Tinsley Harrison, a friend of Alfred Blalock, and future author of “Harrison’s Principles of Internal Medicine”, convinced Blalock to enter instead the general surgical residency at Nashville, Tennessee, under Barney Brooks. Thus, in late 1925, after 3 varied years of surgical training at Hopkins, and without unpacking his belongings in Boston, Blalock journeyed to Vanderbilt University in Nashville to complete his general surgical training. Early on, Barney Brooks placed Blalock in charge of the experimental laboratory at Vanderbilt, as he valued the prior experience Blalock had gained during animal investigations at Hopkins.
In 1927, Blalock contracted tuberculosis, and spent a year in the Trudeau Sanatorium in upstate New York. Depressed with his progress at Trudeau, Blalock left for Europe, and worked for a few months in a physiology laboratory in Cambridge, England. During a sojourn to Germany, Blalock sought advice about his tuberculosis from the then well-known Berlin surgeon, Ernst Ferdinand Sauerbruch. While in Berlin, Blalock suffered a pulmonary haemorrhage. Even so, Sauerbruch ignored a plea by Blalock for help. Fortunately, Blalock recovered without treatment and he left Berlin with an understandable bitterness towards Sauerbruch. Blalock then returned to the Trudeau Sanatorium for treatment. After his recovery, in 1928, Blalock went back to work at Vanderbilt.
Following his residency training, Blalock joined the Vanderbilt faculty, reaching full professor by 1938. With an experimental foundation laid at Hopkins, Blalock built his own animal laboratory at Vanderbilt, where he conducted research, principally related to shock. Over the years, Blalock collaborated with several investigators, including his friend, Tinsley Harrison. The research undertaken by Blalock proved a concept obvious today, but which was then controversial, namely that massive loss of blood leads to shock. His investigations were timely, as they benefited many soldiers wounded during the Second World War.
Vivien Thomas5–Reference Timmermans7
Vivien Thomas (Fig. 2) was born in Louisiana. Shortly afterwards, his family moved to Nashville, Tennessee. Vivien attended a segregated high school, but he hoped to go to college, and eventually to medical school. In 1929, he was working as a carpenter, and attending the Tennessee Agricultural and Industrial State School as a premedical student. His dreams ended with the onset of the Great Depression. In February, 1930, Vivien accepted reality. Without resources, he could not attend medical school. Charles Manlove, a friend of Vivien Thomas, worked as a technician at Vanderbilt. Vivien questioned Charles about jobs at the medical centre. Charles replied that he knew about an opportunity for a technician in the laboratory run by Alfred Blalock. Thomas interviewed with Blalock, and Blalock hired him on the spot. Blalock took great pride in his laboratory work. He must, therefore, have seen something special in Vivien Thomas.

Figure 2 Vivien Thomas (1910–1985).
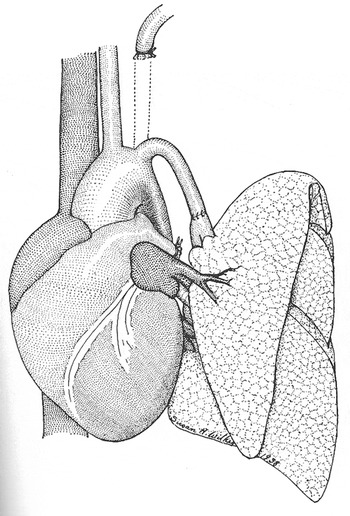
A research fellow working under Blalock, Joseph Beard, became an important mentor to Vivien Thomas during the first year Thomas worked in the laboratory. Beard taught him chemistry, physiology, how to use a slide-rule, and engendered in him an enthusiasm for investigation. For the next 10 years at Vanderbilt, his skills matured, and he assumed a central role in the research laboratory. Thomas performed many animal experiments, and developed various new surgical techniques. Among the experiments that Blalock and Thomas carried out at Vanderbilt, one was specifically noteworthy. In 1938, Blalock, Thomas, along with Sanford Levy, decided to investigate the effects of pulmonary hypertension on the pulmonary parenchyma. They elected to create pulmonary hypertension by anastomosing the subclavian artery, end-to-end, with a branch of the pulmonary trunk (Fig. 3).Reference Levy and Blalock8 Because the model was not a chronic one, they detected no significant pulmonary parenchymal changes using light microscopy. They ceased the experiment, yet still published their negative results. Similar to most of the articles published by Blalock, Vivien Thomas was not included in the list of authors.
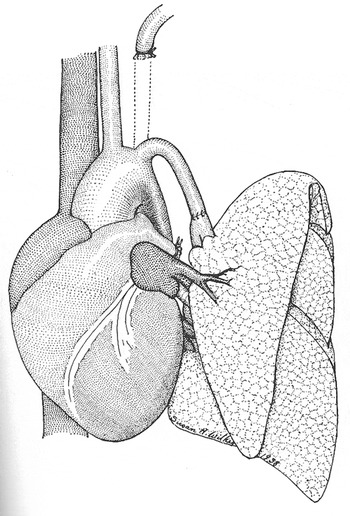
Figure 3 Diagram of the model for pulmonary hypertension created by Blalock, Thomas, and Levy, reproduced from reference #8.
In 1938, the Chairman of Surgery at Johns Hopkins, Dean DeWitt Lewis, had a stroke. Hopkins appointed a search committee that began a protracted process, lasting 3 years, to identify a new chairman. After 2 key individuals declined the position, the committee offered the job to Alfred Blalock. Although the committee preferred their first choices, Blalock coveted the position, and he accepted the offer without hesitation.
Before the offer came from Hopkins, the Henry Ford Hospital in Detroit had offered the Chair of Surgery to Blalock. The hospital administration, however, bluntly told Blalock that Vivien Thomas was not welcome. Blalock was firm. He had no interest in going to Henry Ford if the hospital were to exclude Thomas. Presumably, a practical need for the expertise provided by Thomas prompted the response by Blalock, rather than social conscience.
In July 1941, Blalock embarked on his chairmanship of surgery at Johns Hopkins. Vivien Thomas accompanied Alfred Blalock to segregated Baltimore, where they and Helen Taussig developed a symbiotic relationship, complicated by personality, gender, and race.
Helen Taussig5, Reference Baldwin9–Reference McNamara, Manning, Engle, Whittemore, Neill and Ferencz14
Helen Brooke Taussig (Fig. 4) was born in Boston. Her father, Frank William Taussig, was the distinguished Henry Lee Professor of Economics at Harvard. Her mother was a graduate of Radcliffe, and a biologist. As a young girl, Helen enjoyed the lessons from her mother about the botany of Cape Cod, the location of their summerhouse. Her mother, however, died of tuberculosis when Helen was only 11 years old. Helen then grew closer to her father, who helped her to overcome the dyslexia that plagued her schoolwork.
Figure 4 Helen Taussig (1898–1986).
Like her mother, Helen initially attended Radcliffe as an undergraduate. At Radcliffe, she played tennis and basketball. In 1919, she transferred to the University of California at Berkeley, from which she graduated in 1921. She had become familiar with Berkeley when she accompanied her father one summer when he taught a course in economics there. At Berkeley, she acted in school plays, and in her free time hiked throughout the Yosemite National Park.
Despite protests from her father, Helen wished to apply to medical school. She was rejected by Harvard because they did not accept women, even those who were daughters of famous Harvard Professors. For a couple of years, Helen took premedical courses at both Harvard and Boston University. At Boston University, a Professor of Anatomy, Alexander Begg, became an inspirational mentor. Helen worked with him on research into the myocardium, which she later published in the American Journal of Physiology, her first contribution to the medical literature.Reference Taussig and Meserve15 Alexander Begg counselled Helen to apply to Johns Hopkins, as the institution had a policy of admitting women, this being the legacy of a group of women donors who helped found the Johns Hopkins School of Medicine in 1893.Reference Bernheim16
Accepted at Hopkins, Helen pursued her interest in cardiology that began with Alexander Begg by signing up for an elective in the adult heart station. There, she fell under the spell of her next mentor, Edward Perkins Carter. Following graduation in 1927, Helen failed to obtain an internship, and Edward Carter invited her to spend an extra year learning cardiology. In 1928, another important mentor, Edwards Park, assumed the Chair of Paediatrics at Hopkins. Following a year in the heart station, Taussig completed a paediatric residency under Edwards Park. The visionary Park decided to set up the first paediatric specialty clinics in North America. He initially asked Clifton Briggs Leech to head the cardiology clinic.Reference Evans17 Leech left in 1930, and Park appointed his junior faculty member, Helen Taussig, fresh out of residency, to be the new head of the cardiology clinic.
Development of the systemic-to-pulmonary arterial shunt
Following the report of ligation of the arterial duct by Gross and Hubbard, but probably before the arrival of Alfred Blalock in Baltimore in 1941, Helen Taussig ventured to ask Robert Gross, the Chief of Surgery at Boston Children’s Hospital, if he would create an artificial duct for her patients with tetralogy of Fallot. Neither the Alan Mason Chesney Medical Archives at Johns Hopkins, nor the archives of Boston Children’s Hospital, possess correspondence between Taussig and Gross to help pinpoint the date of her visit to Boston.18
In 1979, Childrens Hospital of Los Angeles held a symposium that included Helen Taussig as an honoured guest. A cardiovascular surgeon at the host facility, Bernard Tucker, compiled the proceedings of the meeting into the “First Clinical Conference on Congenital Heart Disease” (Fig. 5).Reference Tucker, Lindesmith and Takahashi19 The meeting concluded with a dinner, during which a fascinating conversation, preserved in the book edited by Tucker, occurred between Helen Taussig and Louis Martin, one of her old mentors from John Hopkins, and a former director of cardiology at the host institution. During this conversation, Helen relayed, “Dr Gross tells this story on himself, so it is all right for me to tell it on him. I went to him and asked if he could build a ductus.” Helen reported that Dr Gross replied, “Oh, yes, I’ve built lots of ductuses. That’s quite easy.” Helen continued, “I very meekly said it would be a great help to a cyanotic child. [Gross] wasn’t a bit interested, so I went back to Baltimore to bide my time.” The response by Gross to Taussig has been popularly quoted as, “Madam, I close ductuses, I don’t create them.”Reference Gott20 But the conversation recorded from Taussig, at the dinner in 1979, was just the opposite. I believe the exchange as written in 1979 was the more accurate portrayal.

Figure 5 Helen Taussig and Louis Martin conducting dinner conversation at Children’s Hospital Los Angeles in 1979.
The response of Gross to Taussig was rooted in an article published by Gross and his colleagues in 1941.Reference Eppinger, Burwell and Gross21 In this article, Robert Gross and his colleagues, physiologist Eugene Eppinger, and the Dean of Harvard Medical School, C. Sidney Burwell, reported experiments in animals where they anastomosed a subclavian artery to the pulmonary trunk to simulate patency of the arterial duct (Fig. 6). This study investigated the physiologic effects stemming from an arterial duct. Their experimental data suggested that blood flowed through the duct from aorta to pulmonary arteries, that the left ventricle received excessive blood from increased pulmonary venous return, and that the arterial blood run-off into the lungs resulted in less flow of blood to other organs.
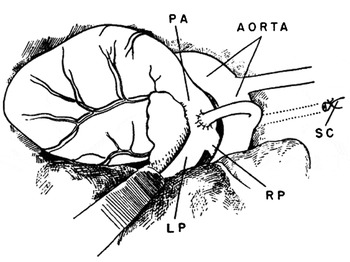
Figure 6 Diagram of the experimental model of an arterial duct created by Robert Gross and colleagues.Reference Eppinger, Burwell and Gross21 PA-pulmonary artery, SC-subclavian artery, RP-right pulmonary artery, LP-left pulmonary artery.
The experiments performed by Robert Gross on the effects of artificial arterial ducts were remarkable. The article may or may not have been available to Taussig when she visited Gross to seek his help in creating an arterial duct. The experiments, nonetheless, clarified the response by Gross to Taussig over creating artificial arterial ducts.
Reportedly, Taussig briefly considered moving to Boston had Gross agreed to the concept of creating an artificial duct. An alternate history may have transpired had Robert Gross grasped the anatomy and physiology of tetralogy of Fallot like Helen Taussig, possibly resulting in a Gross-Taussig shunt rather than the Blalock-Taussig shunt. In 2006, W. Hardy Hendren, a former surgical resident trained by Gross, wrote, “Gross regretted that he had not paid heed to Helen Taussig who had visited him earlier from Hopkins and suggested ‘making a ductus’ as treatment for blue babies.”Reference Hendren22 The experimental model used by Gross was the forerunner of the central systemic-to-pulmonary arterial shunt.
Following the appointment of Alfred Blalock as Chief of Surgery at Johns Hopkins in 1941, he ligated many patent arterial ducts. Quoting again from the conversation between Louis Martin and Helen Taussig at the dinner in 1979, Louis Martin recounted, “Following on Dr Blalock’s patent ductus operations, Dr Taussig was standing at his elbow, and she said, ‘Dr Blalock, you’ve done a very nice job closing this ductus; why can’t you build a ductus? We need it for our cyanotic children. To some of our cyanotic children, it would mean a life for them’.”Reference Tucker, Lindesmith and Takahashi19
Simultaneous to the growing experience with surgery on the arterial duct, Edwards Park proposed a plausible solution for coarctation of the aorta,Reference Helen23 making the suggestion before Swedish surgeon, Clarence Crafoord, first repaired aortic coarctation in 1944 and 1945.Reference Crafoord and Nylin24 Park recommended to Blalock that he transect the subclavian or carotid artery and anastomose the vessel below the coarctation so as to bypass the obstruction. The idea intrigued Blalock. Thus, he and Thomas began experiments in animals to test the method of repair proposed by Park. Blalock and Thomas initially created an artificial coarctation. They followed the experimentally created coarctation by a turning down, and anastomosing in end-to-side fashion, either a carotid or subclavian artery to bridge the narrowing. In the dog, Blalock and Thomas found the subclavian artery to be more suitable for the desired purpose than the carotid artery.Reference Blalock and Park25 The animals did poorly, and Blalock never used this operative technique in humans, although the idea later led to the development of the subclavian flap repair for coarctation.
The experiments in animals, attempting physiologically to correct coarctation by turning down the subclavian artery, also stimulated Taussig to believe that her theory of an artificial arterial duct could be practically implemented. Again, in the conversation with Louis Martin recorded in 1979, Taussig explained, “Dr Park also tried to get Dr Blalock to correct coarctation of the aorta. It is interesting that when the aorta in a normal dog is cross-clamped, 50 percent will get paralysis of the lower extremities and 50 percent will not. It so happened that Gross’s dogs did not [referring to Boston’s Robert Gross and his animal coarctation experiments] and Blalock’s dogs did. So Blalock had to find another way. Dr Park said, ‘The carotid artery is a long, straight vessel; can’t you take that down and bypass the coarctation?’ Blalock said, ‘Um-hm.’ I said, ‘Well, if you can take the carotid down, couldn’t you take the subclavian artery and put it into the pulmonary artery? That’s all I want you to do.’ He invited me over to the laboratory, and I started to work with Vivien Thomas.’”Reference Tucker, Lindesmith and Takahashi19
In the autobiography by Vivien Thomas published in 1985, “Pioneering Research in Surgical Shock and Cardiovascular Surgery”, Vivien reported the visit by Helen Taussig to the Hunterian laboratory in 1943, and the protracted conversation she had with Blalock and him. Vivien Thomas wrote, “Helen passionately described her patients and their plight and that no known medical treatment existed. She went on to suggest that their only hope was a type of surgical approach to ‘get more blood to the lungs, as a plumber changes the pipes around.’”Reference Thomas26 From the account reported by Thomas, Blalock agreed with the course advocated by Taussig. In his book, Vivien Thomas conveyed he lacked knowledge of possible previous conversations between Taussig and Blalock over a surgical approach to tetralogy of Fallot. His firsthand report, nonetheless, supports the account that Helen Taussig launched the research to find a palliative surgical treatment for patients with tetralogy of Fallot.
Blalock directed Thomas to develop an experimental model of an animal with cyanosis, followed by a second procedure to ameliorate the experimentally created cyanotic state. Vivien Thomas spent about a year trying to develop such an animal model with decreased pulmonary flow. The final model included anastomosing a pulmonary artery with a pulmonary vein, combined with partial resection of the lung. Once the model had been established, Thomas then proceeded to anastomose a subclavian artery to the pulmonary artery, leading to a slight increase in effective pulmonary flow, resulting in a decrease in cyanosis. The subclavian artery-to-pulmonary arterial connection was a modification of the model to produce pulmonary hypertension developed by Blalock, Levy, and Thomas at Vanderbilt in 1938. The earlier jump-graft experiment for a potential treatment of coarctation also influenced the development of the subclavian-to-pulmonary arterial shunt.
Between the fall of 1943 and 1944, Vivien worked on refining the operation in animals. In late November 1944, Blalock had assisted Thomas in only one animal experiment, but Blalock, acting as the primary surgeon, had yet to perform the procedure in an animal subject. In his autobiography Thomas wrote, “Dr Blalock said that he was going to have to learn to do the subclavian artery-to-pulmonary artery anastomosis, so that he could perform the surgery on a patient. He wanted to assist me in doing one on a dog and then do one or two with my assistance.” Vivien further stated, “He came as scheduled and assisted.” Vivien went on, “On the day he was scheduled to perform the procedure [on an animal], he telephoned early in the morning. The condition of the patient on whom he planned to operate was deteriorating so rapidly that he could not delay the procedure.”Reference Thomas26
Under orders from Blalock, Vivien Thomas worked closely with the staff to ready the operating room for the first surgery on a child. Tools were primitive, and the instruments and sutures surgeons take for granted now were then unavailable. Thomas, however, had fashioned a special vascular clamp for the operation (Fig. 7).5 On November 29, 1944, the nurses brought fragile, frightened, cyanotic 15-month-old Eileen Saxon to the operating room. During the surgery, Blalock insisted that Thomas stand at his side to guide his hands and offer advice. Helen Taussig was in the operating room also, and she stood where she could see Eileen’s face, next to the anaesthesiologist, Merel Harmel.Reference Blalock and Taussig27

Figure 7 The vascular clamp designed by Vivien Thomas in collaboration with William Longmire and the Baltimore surgical supply company Murray Baumgartner & Co.
Ruth Whittemore, then a paediatric resident at Hopkins, assisted Helen Taussig in the postoperative care for Eileen Saxon. Ruth Whittemore graduated from Johns Hopkins Medical School in 1942 and interned at Yale, returning to Johns Hopkins for her paediatric residency. In a quotation from “The Developing Heart: A ‘History’ of Pediatric Cardiology”, by Neill and Clark, Ruth Whittemore described the postoperative care, “[Eileen Saxon] came out of surgery with numerous postoperative complications. I had to stick needles into both sides of her chest to draw off air that was compressing her lungs. We didn’t have a way to monitor the pressure on her lungs, so I rigged up a way to monitor it without having to stick her repeatedly. It was definitely a Rube Goldberg affair, but the baby came through it. As for me, I fell into pediatric cardiology and couldn’t get out.”Reference Neill and Clark28 Whittemore stayed at Hopkins, serving as the first paediatric cardiology fellow under Helen Taussig. The many fellows in paediatric cardiology who followed Ruth Whittemore fell under the spell of Taussig. Even after the fellows left Hopkins, they remained devoted to their mentor, calling themselves “The Knights of Taussig.”Reference Bart29
In 1947, 3 years after the first procedure, Alfred Blalock and Helen Taussig triumphantly toured Europe without Vivien Thomas. They lectured about the procedure, and Blalock operated on 10 children in London, at Guy’s Hospital, assisted by Russell Brock. Following the lecture given by Blalock and Taussig in London, Brock described the scene, “The hall was quite dark for the projection of his [Blalock’s] slides when suddenly a long searchlight beam traversed the whole length of the hall and unerringly picked out on the platform a Guy’s nursing sister, dressed in her attractive blue uniform, sitting on a chair holding a cherub-like girl of two and a half years with a halo of blonde curly hair and looking pink and well; she had been operated on at Guy’s by Blalock a week earlier. The effect was dramatic and theatrical and the applause from the audience was tumultuous.”Reference Hurt, Barry, Adams and Fleming30
Controversy concerning the eponym
Alfred Blalock delivered a Harvey Lecture, before New York City’s Harvey Society, in November 1945, 1 year after the creation of the first systemic-to-pulmonary arterial shunt. In this lecture Blalock said, “Following the original suggestion of Dr Taussig some two years ago that a means be found for increasing the flow of blood to the lungs, I undertook studies on experimental animals. It was evident that there were two major problems, namely, (1) the devising of a technique by which the blood flow to the lungs could be increased, and (2) the testing of the method in animals with a high degree of unsaturation of the arterial blood.”Reference Blalock31 Thus, in this important forum, contemporary to the first shunt procedures, Blalock acknowledged the fundamental insight made by Helen Taussig.
The first appearance in the literature of “the Blalock-Taussig operation” occurred in 1946 as part of a discussion following a presentation by Alfred Blalock to the American Surgical Association at their meeting in April of that year in Hot Springs, Virginia.Reference Blalock32 “Blalock-Taussig shunt” did not appear in the literature until 1966. Decades later, however, others expressed opinions suggesting that not everyone accepted the eponym.
Richard Bing arrived at Johns Hopkins after the Blalock-Taussig shunt had undergone experimental development and clinical use. Prior to arriving at Johns Hopkins, Bing taught physiology at Columbia University before the end of the Second World War. In 1945, Alfred Blalock invited Bing to join the Hopkins faculty. Blalock realized the importance of acquiring invasive physiologic information on patients with congenital cardiac malformations prior to surgery, and Blalock recruited Bing to carry out this task.Reference Bing, Vandam and Gray33 Vivien Thomas assisted Richard Bing in their first cardiac catheterizations at Hopkins. A former paediatric patient, Sandra Stoltz, now a psychologist in Arizona, quoted in an accompanying article to the film “Partners of the Heart”, produced in 2003 about Blalock, Thomas, and Taussig, recounted, “I met Dr Taussig when we came to Hopkins to be evaluated for surgery.… She struck me as a very kindly, soft-spoken lady and I liked how she talked to me. She didn’t talk down to me.… I remember her being very reassuring and her calm, quiet voice was very soothing. [Blalock] was more remote; he was like God. The big surgeon. He was a very handsome man and he seemed big to me. I don’t know whether he actually was or not. But I liked him. I really liked him. I remember Vivien Thomas as this very kindly, gentle, soothing man. I don’t know what exactly he did to me; I think it was some sort of angiogram. He described the procedure step-by-step. He told me exactly what to expect and he made it unscary. He really did. He created a profound impact and of course, as an adult psychologist, I know he was way ahead of his time in terms of how to prepare children for something like this.”Reference Stoltz34
Decades afterward, Richard Bing wrote, in 1992, “Cardiology: the Evolution of the Science and the Art”.Reference Bing35 In this book, Bing briefly outlined the history of paediatric cardiology and paediatric cardiac surgery, supplemented by his firsthand experiences and recollections. Similar to other authors, in discussing the history of cardiac surgery, Bing addressed his opinion about the “authorship issue” of the Blalock-Taussig shunt. Surgeons tend to side with Blalock, as paediatric cardiologists do with Taussig. Bing was then neither a surgeon nor a paediatric cardiologist but a skilled cardiac physiologist. In his book, Bing lauded the physiologic approach undertaken by Blalock towards care, and he subtly implied its superiority to the clinical approach advocated by Taussig. Richard Bing wrote, “It is certain, however, that Helen Taussig’s idea that an increase in pulmonary blood flow would be beneficial was not [emphasis added] correct. It was not the diminution in pulmonary blood flow, but the right to left shunt, or the decrease in effective pulmonary blood flow, which was responsible for the cyanosis and clinical symptoms of these patients.”
Notwithstanding the significant contribution on an obligatory right-to-left shunt to cyanosis, increasing flow of blood to the lungs by creating a systemic-to-pulmonary arterial shunt in a cyanotic patient with tetralogy of Fallot does slightly increase effective pulmonary flow. The increase in effective pulmonary flow results in an increase in desaturated blood exposed to alveoluses, leading to an improvement in saturations of oxygen. Thus, the original idea developed by Taussig proved correct both physiologically and clinically.
In the book written by Bing, he also quoted from the 1991 biography of Blalock written by William P. Longmire. Longmire was a protégé of Blalock, and Chairman of Surgery at University of California at Los Angles from 1948 to 1976. Longmire wrote, “Taussig’s role on the tetralogy of Fallot event has been the subject of some controversy and has been enlarged or diminished, in part, depending upon whether the narrator is a surgeon or a cardiologist…[it was] only because of Blalock’s custom of giving generous recognition to his associates that Helen Taussig began to receive a share of the acclaim.”Reference Bing35 As a senior resident, William Longmire attended the first procedure Blalock performed in November 1944.36 Longmire, however, was likely unaware of previous discussions between Taussig and Blalock, or the visit Taussig undertook to speak with Robert Gross. The account outlined by Longmire in his biography of Blalock contradicted historical accounts. Helen Taussig’s grasp of clinical, pathological, and physiological findings in congenital heart disease originated from her experiences with patients, and from her close association with the early 20th century congenital cardiac morphologist, Maude Abbott.Reference Evans37 These experiences, occurring over a decade, led Taussig to develop the theory of creating an artificial arterial duct.
Harris B. Shumacker, yet another pioneering cardiac surgeon trained by Alfred Blalock, worked with the cardiology programme at Riley Children’s Hospital in Indiana, where he was the Chairman of the Department of Surgery from1948 to 1968.38 Shumacker also weighed in on the “Blalock-Taussig shunt” term. In 1992, Shumacker wrote, “The Evolution of Cardiac Surgery”.Reference Shumacker39 He titled chapter 10, “The Blalock Operation Systemic-Pulmonary Shunts.” Shumacker removed “Taussig” from the eponym, and he enigmatically wrote, “Those who knew Blalock understood why he insisted that the original publication state clearly that the idea of the operation was Taussig’s.” Shumacker went further, “It is equally evident that she [Helen] would not have made such a proposal had it not been for her colleague’s extensive experience with arterial anastomosis. Blalock had produced experimental cyanosis in dogs and had shown that a systemic-to-pulmonary arter shunt provided them with better oxygenation. The experiments were carried out before the first clinical operation, but the results were not published until after the clinical report appeared.” But Taussig did not propose an operative approach because of prior experimental efforts by Blalock. The experimental techniques that Vivien Thomas devised and carried out resulted from the discussions in 1943 between Taussig, Thomas, and Blalock about a surgical method for treating cyanotic children by improving the flow of blood to the lungs.
Conclusions
Historical records support the premise that Helen Taussig originated the concept of an ameliorating artificial arterial duct. Its application, however, required the talents of Vivien Thomas and Alfred Blalock, culminating in the onset of an undisputed revolution for patients suffering cyanosis secondary to obstructed pulmonary blood flow. Thus, credit belongs to all 3, with each contributing their unique part. But as Brogan and Alfieris noted in a similar article on the history of the Blalock-Taussig shunt, it would likely be impractical now to change the eponym to include all 3 names, despite the central part Vivien Thomas played in effecting the practical goal of applying the systemic-to-pulmonary arterial shunt in patients.Reference Brogan and Alfieris40
To the best of my knowledge, no other current, frequently performed, congenital cardiovascular surgical procedure labelled by an eponym includes the name of a paediatric cardiologist, rather than exclusively the names of cardiovascular surgeons. The eponym “Blalock-Taussig shunt” should also serve as a reminder of the importance that collaboration between congenital cardiologists and cardiovascular surgeons brings to the care of patients with complex anatomical and physiological abnormalities. In the current era, one would also hope that the essential contributions of those providing not only technical assistance, but also intellectual input, would not be ignored.Reference Murphy and Cameron41 Although the eponym for the shunt does not include the deserving name of Vivien Thomas, he eventually received accolades due him, including an honorary doctorate from The Johns Hopkins University in 1976 (Fig. 8).5

Figure 8 Vivien Thomas with Helen Taussig, and Steven Muller, President of The Johns Hopkins University, at graduation ceremonies in 1976, during which Thomas was honoured.