Percutaneous balloon dilation is an established initial procedure for relief of neonatal critical aortic valvar stenosis.Reference Wren, Sullivan, Bull and Deanfield1–Reference Weber3 Various techniques have been reported for this procedure, albeit that currently there is no agreement regarding the optimal vascular approach. The umbilical arterial approach has been reported in only a few patients.Reference Beekman, Rocchini and Andes4, Reference Egito, Moore and O’Sullivan5 Since these initial reports, catheter technology has markedly improved,Reference Kim, Raviele and Vincent6 but reports using the 3 French balloon catheters now available are lacking for the umbilical approach. In this report, therefore, we describe our own experience using 3 French balloon catheters to achieve balloon dilation of critical aortic valvar stenosis in neonates via the umbilical artery.
Methods
Since June of 2005, we have attempted to obtain umbilical arterial access within the first week after birth in all neonates presenting with critical aortic valvar stenosis. All such patients have been included in the present report, and in all we attempted balloon dilation using this approach. The retrospective analysis of the data was approved by an authorized ethics committee.
Catheterization of the umbilical vessels was performed in the neonatal intensive care unit. A 5 French Argyle umbilical catheter (Tyco Healthcare, Tullamore, Ireland) was inserted into the umbilical vein, and a 3.5 French Argyle umbilical catheter into the umbilical artery. The tip of the arterial catheter was positioned in the mid-thoracic component of the descending aorta. Both catheters were tied in place with a silk suture, which was taped to the abdominal wall, and the catheter positions were then verified by X-ray. The venous catheter was used to provide a continuous infusion of prostaglandin. Balloon dilation was performed in the cardiac catheterization laboratory under general endotracheal anaesthesia. Cefazoline at 20 mg/kg was administered intravenously before the procedure, and at 6 and 12 hours after the procedure. The umbilical area was prepared and draped in standard sterile fashion, and the positions of the catheters further confirmed by fluoroscopy (Fig. 1). A floppy-tipped 0.014 coronary guide wire was inserted through the umbilical arterial catheter, with the floppy tip curved above the aortic valve (Fig. 2). The catheter was then removed, and replaced by a 4 French Terumo sheath 5 cm in length (Terumo Corporation, Tokyo, Japan). No heparin was given. A 4 French Super Torque multi-purpose A2 catheter (Cordis Corporation, Miami, FL) was positioned into the ascending aorta, and an aortogram performed by hand injection of contrast. The aortic valve was crossed using a floppy-tipped 0.014 coronary guide wire. Tyshak Mini balloon dilation catheters (NuMED Canada Inc., Cornwall, ON) were used in all patients for dilating the stenotic valve. The diameter of the balloon was selected so as to be approximately equal to the diameter of the aortic valve as measured by echocardiography. Balloon dilation was performed by hand inflation using saline-diluted contrast. After dilation, pressures were measured and the aortogram was repeated. The results were assessed by transthoracic echocardiography performed in the cardiac catheterization laboratory. Haemostasis was subsequently obtained by digital pressure on the umbilical artery.
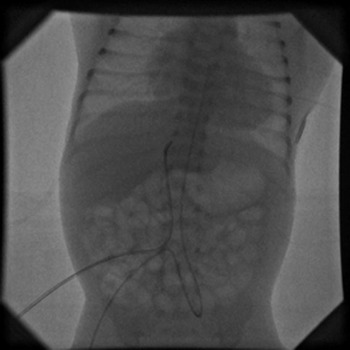
Figure 1 5 French and 3.5 French umbilical catheters are placed into the umbilical vein and artery in a 1.8 kg newborn.

Figure 2 A floppy tip of a 0.014 coronary guide-wire is curved above the aortic valve. A guide-wire is inserted through the 3.5 French umbilical artery catheter.
Results
Since June of 2005, we were presented with 5 neonates diagnosed with critical aortic valvar stenosis within the first week of birth. All patients at presentation were in congestive cardiac failure or shock. An infusion of prostaglandin was started in all following the initial echocardiographic evaluation. We were able to obtain umbilical arterial access in all, and in all we then attempted balloon dilation via the umbilical artery. The clinical and procedural data is presented in Table 1. Our second patient was a twin born after 35 weeks of gestation weighing 1.8 kg. In the third patient, umbilical arterial access was secured with the tip of a 3.5 French umbilical vessel catheter positioned in the mid-thoracic aorta. A floppy tip of a 0.014 coronary guide wire was positioned in the ascending aorta, and the umbilical catheter exchanged for a 4 French sheath. The tip of the sheath was placed in the umbilical artery proximal to its junction with the internal iliac artery. We were unable to negotiate either a 4 French multi-purpose catheter or a 3 French Tyshak Mini balloon dilation catheter into the internal iliac artery. The stenosis, therefore, was effectively relieved via the femoral artery. In the fifth patient, critical aortic valvar stenosis was detected during the 35th week of gestation. After corticosteroid preparation, the patient was born by caesarean section weighing 2.2 kg. Echocardiography performed after birth revealed severe aortic coarctation in addition to critical aortic valvar stenosis.
Table 1 Summary of clinical and procedural data.

FA = femoral artery; UA = umbilical artery; ↓LV = mild impairment of left ventricular systolic function; ↓↓LV = severe impairment of left ventricular systolic function.
The acute and follow-up data is summarized in Table 2. There were no procedural deaths or complications related to the procedure. The stenosis was effectively relieved in all patients. In patients with severe impairment of left ventricular systolic function, we relied on the valvar morphology and function, rather than the measured gradient, to confirm relief of stenosis. Loss of blood was negligible in all patients. Haemostasis was easily achieved in all patients at the end of the procedure. In 3 patients, further procedures were necessary, specifically re-dilation of the stenotic aortic valve, the Norwood procedure, and repair of aortic coarctation. Of the patients, 2 are alive and well 42 months and 7 months after the initial dilation. In the third patient, systolic left ventricular function normalized immediately after the dilation, and extubation was possible within 24 hours. He was discharged home and his progress during the first month was satisfactory. Thereafter, progressive diastolic dysfunction developed causing pulmonary congestion and pulmonary oedema. At the age of 2 months, the patient underwent the Norwood procedure using a ventriculo-pulmonary shunt, with simultaneous over-sewing of the aortic and mitral valves. He suddenly suffered cardiac arrest 2 days after surgery and could not be resuscitated. The fifth patient underwent repair of aortic coarctation along with an attempt at surgical valvotomy. Direct inspection of the aortic valve confirmed effective relief of the stenosis by balloon dilation.
Table 2 Summary of acute and follow-up results.

AR = aortic regurgitation; CoA = coarctation of aorta; N = normal left ventricular systolic function; ↓↓LV = severe impairment of left ventricular systolic function.
Discussion
Critical aortic valvar stenosis in neonates most often presents soon after birth with signs of congestive cardiac failure or shock. Percutaneous balloon dilation is most frequently used for immediate relief of the stenosis, and various vascular approaches have been advocated for this purpose.Reference Weber3 There is no current agreement, however, regarding the optimal approach. The umbilical arterial approach has been reported, but thus far in few patients.Reference Beekman, Rocchini and Andes4, Reference Egito, Moore and O’Sullivan5 In one of these reports, a cohort of 4 patients was described.Reference Beekman, Rocchini and Andes4 Access was obtained via the umbilical artery in neonates aged from 2 to 11 days, and dilation achieved successfully in all. Catheters of 5 French dimensions were introduced into each umbilical artery, and 4 French or 5 French catheters into the umbilical vein across the oval foramen into the left ventricle. At that time, they used 5.3 French balloon catheters inserted over 0.035 guide wires. An episode of sepsis was reported after the procedure in one patient. Then another groupReference Egito, Moore and O’Sullivan5 reported on 11 patients treated within 72 hours of birth, albeit that details of the technique were not described. If it proved impossible to cross the aortic valve within 30 minutes, balloon dilation was performed via the femoral artery. A procedural death occurred secondary to sepsis following a prolonged attempt at dilation via the umbilical artery.
We now describe effective relief of critical aortic valvar stenosis in 4 neonates via the umbilical artery using 3 French balloon catheters. To minimise the time spent in the catheterization laboratory, we inserted the catheters in the umbilical vessels in the neonatal intensive care unit. We obtained access to the umbilical artery of a neonate of 3 days using a 3.5 French umbilical catheter. We were unable, however, to negotiate a 4 French multi-purpose catheter and a 3 French balloon dilation catheter into the internal iliac artery. During this manoeuvre, we avoided using undue force to prevent injury of the umbilical artery or internal iliac artery. After only a short additional time in the cardiac catheterization laboratory, we realized that dilation via the umbilical artery was not feasible. In the remaining 4 patients, the procedure proved remarkably safe, simple, and quick. The dilation via the umbilical artery was achieved in patients weighing 1.8 and 2.2 kg. Previously, the lightest patient reported has been 3.3 kg.Reference Beekman, Rocchini and Andes4 We now demonstrate that dilation using currently available catheters inserted through the umbilical artery is also feasible in neonates weighing less than 2.5 kg. The simplicity of the procedure is evident from our short times required for fluoroscopy, which ranged from 6 to just over 15 minutes. The longest time was needed in a patient weighing 2.2 kg who also had severe aortic coarctation, the latter lesion significantly prolonging the time required for fluoroscopy. In comparison, the times required using catheters of 5.3 French dimensions ranged from 15 to 38 minutes.Reference Beekman, Rocchini and Andes4 Because of the shortness of our procedures, we opted not to use heparin. We chose balloons of equal diameter to the aortic valvar hinges in all our patients, all of whom had impaired left ventricular function before the procedure. In this way, the left ventricular pressure overload was decreased as much as possible to allow recovery of myocardial function without creating excessive aortic regurgitation.
The retrograde femoral arterial approach is currently used most frequently for balloon dilation of neonatal critical aortic valvar stenosis, probably because the technique is simple and straightforward. Accessing the femoral artery in neonates, however, may be difficult and time-consuming due to diminished or absent femoral pulses. Furthermore, using currently available catheters, the risk of femoral arterial injury is reduced but not eliminated. Once vascular access is secured, the procedure is practically identical when using either the umbilical arterial or the femoral arterial approach. An experience using the femoral arterial approach was reported recently in 20 neonates and infants less than 6 months of age.Reference Kim, Raviele and Vincent6 As with our current report, a 3 French system was used in all of their patients. They noted their mean procedural time, but excluded the time required to obtain arterial access. There was no clinical evidence of an arterial complication in any of their patients. Five patients underwent subsequent cardiac catheterizations. Femoral arterial occlusion was detected in one, albeit not ascribed to the balloon valvoplasty performed in the neonatal period.
The transvenous antegrade approach is technically most demanding.Reference Magee, Nykanen, McCrindle, Wax, Freedom and Benson7 It does, however, eliminate the risk of femoral arterial compromise. In addition, it avoids the possibility of inadvertent perforation of an aortic valvar leaflet, and increases the stability of the balloon during dilation, thus causing less aortic regurgitation. The integrity of the atrial septum, and the presence of a hypoplastic and hypertrophied left ventricle contribute to the technical difficulties of the procedure, particularly in neonates. Damage to the mitral valve may occur should the balloon catheter be inadvertently looped towards the left ventricular apex during dilation.
The right carotid arterial approach via a direct surgical cut-down was also developed to avoid femoral arterial compromise.Reference Fischer, Ettedgui, Park, Siewers and del Nido8 This approach simplified the retrograde passage across the aortic valve. The procedure can also be performed at the bedside under continuous transoesophageal echocardiographic guidance.Reference Weber, Mart and Myers9 Compromise of the carotid artery is currently reduced by using balloon catheters with low profiles. Cerebral lesions, nonetheless, remain an ongoing concern using this approach.
In conclusion, we believe that the umbilical arterial approach should always be considered for balloon dilation of neonatal critical aortic valvar stenosis. Using currently available catheters, the procedure is safe, simple, and effective even in patients weighing less than 2.5 kg. Further experience using this vascular approach is therefore warranted.
Acknowledgements
T.P. was supported in part by the Slovenian Agency for Research grant # J3-9663.






