Aortic coarctation in infants with low birth weight presents a therapeutic dilemma. Although surgery is the standard treatment for native coarctation in neonates, it carries a high risk of complications in low-birth-weight infants such as potential injury to the immature central nervous system, the development of restenosis, and death.Reference Dryzek, Goreczny and Kopala1 The high risk of morbidity and mortality with surgery in low-birth-weight infants required a need for catheterization-based interventions. Balloon angioplasty was used to delay the surgery. In the literature, the number of cases less than 2000 g undergoing balloon coarctation angioplasty is limited.
Case 1
A male born at the gestational age of 32 weeks with birth weight 1490 g complained of respiratory distress. His weight was 1570 g during the procedure. The arterial oxygen saturation was 97%. Echocardiography showed patent ductus arteriosus, aortic coarctation, secundum atrial septal defect, and moderate tricuspid valve regurgitation. Arterial blood pressures were 80/34/49 mmHg on right arm, 60/31/43 mmHg on right leg, before the intervention and 82/56/63 mmHg on right arm, 74/46/54 mmHg on right leg, after the intervention. We thought that the baby could not tolerate surgical coarctation repair. He was prepared for balloon angioplasty.
Case 2
A female neonate was born as the first twin in the 32nd week of gestation. Her weight was 1450 g at birth, and 1520 g during the intervention. She was referred to our clinic for respiratory distress. Echocardiography revealed aortic coarctation and patent ductus arteriosus. Arterial blood pressures were 92/48/65 mmHg on right arm, 53/38/44 mmHg on right leg, before the intervention and 91/58/76 mmHg on right arm, 95/54/73 mmHg on right leg, after the intervention. Balloon angioplasty was decided to be performed.
All patients were intubated before the procedure and was administered with general anaesthesia during the procedure. The patients were accessed without difficulty via right femoral artery. Intravenous heparin 50 U/kg was administered after sheath insertion. Contrast material was injected manually into the proximal area of coarcted segment and angiographic views were taken in anteroposterior and lateral before and after the procedure. The coarcted segment and descending aorta at the level of diaphragm measured 1.6 and 5.1 mm in diameter, respectively, for case 1 and 1.5 and 4.9 mm in diameter, respectively, for case 2.
The materials used in the interventional procedures in low-birth-weight infants should be selected carefully to reduce complications related to catheterisation. We thus chose 21 gauge (0.021 inch) needles, 4 F sheath, 4 F multipurpose catheter, 0.014-inch guidewire, and NC Solarice 5 mm × 15 mm balloon catheter (Medtronic, Minnesota, United States of America). The balloon diameter was chosen to be equal to the descending aorta at the level of the diaphragm. Balloon diameter to coarcted segment were 3.1 and 3.3 for case 1 and case 2, respectively. We expanded the balloon with a pressure of 10–12 atmospheres for 5–6 seconds for one–three times under fluoroscopy, until the balloon expands fully, the balloon waist formation appears and this indentation disappears (Figs 1a–c and 2a–c). The site of stenosis was dilated to 2.5 mm and the systolic pressure gradient decreased from 20 to 8 mmHg in case 1, and the site of stenosis was dilated to 2.6 mm and the systolic pressure gradient decreased from 22 to 6 mmHg in case 2. The fluoroscopy and cineangiography times were 14 minutes and 15 seconds, respectively, for case 1 and 12 minutes and 14 seconds, respectively, for case 2. We did not observe any complications, such as pseudoaneurysm or extravasation. Heparin infusion was continued at the dose of 20 U/kg/h for at least 4 hours. Captopril was initiated in both cases after the procedure. Arterial Doppler ultrasound examinations of both patients, performed after the procedure, were normal. There was no difference in blood pressure between upper and lower extremities and no problem in leg length and pulsations of the bilateral lower extremity, during follow-up. Resection of the coarcted segment and end-to-end anastomosis were performed in both cases 5 months after the procedure, due to recoarctation.

Figure 1. (a) Angiographic view of coarctation of aorta in case 1, before angioplasty. (b) Balloon angioplasty of coarctation in case 1. (c) Angiographic view of coarctation of aorta in case 1, after angioplasty.
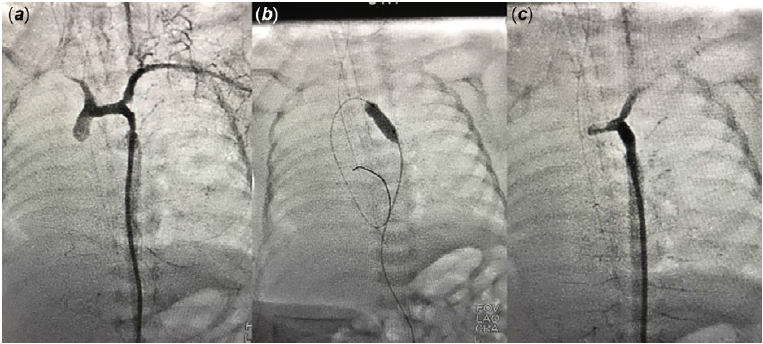
Figure 2. (a) Angiographic view of coarctation of aorta in case 2, before angioplasty. (b) Balloon angioplasty of coarctation in case 2. (c) Angiographic view of coarctation of aorta in case 2, after angioplasty.
Discussion
Balloon angioplasty for aortic coarctation may be considered as an alternative treatment in low-birth-weight infants. Experience with the balloon in infants under 2000 g is limited.Reference Dryzek, Goreczny and Kopala1–Reference Kanagawa, Inamura and Tominaga7 Interventional procedures in low-birth-weight infants have become easier due to the advances of catheterisation techniques and equipment.Reference Karagöz, Akın and Aykan6 The most difficult problem for low-birth-weight infants is the vascular access. It has been reported that the internal carotid or the umbilical arteries could be used. Intervention through these arteries may infarct brain vessels or cause a thrombosis of an abdominal artery.Reference Kanagawa, Inamura and Tominaga7 The use of the femoral artery may interrupt the blood flow to the lower half of the body.
Balloon coarctation angioplasty may be a high risk, but potentially a life-saving procedure in low-birth-weight infants. The risk of complications varies from 0 to 17%.Reference Dryzek, Goreczny and Kopala1 As the morbidity and mortality of the surgery were considered high in these cases, balloon angioplasty was performed in our cases. It was considered that interventional therapy could give high-risk patients time to grow up, to reach a body weight that will allow an appropriate surgery, and to become more stable clinically.
Non-compliant balloons may be preferred for use in small vessels where compliant balloons is not suitable. In a case report published by Dryzek et alReference Dryzek, Goreczny and Kopala1, a compliant balloon was first attempted in an extremely low-birth-weight infant with coarctation of the aorta. However, since they did not observe sufficient expansion in the coarctation area, non-compliant balloon was used in the second attempt. Kanagawa et al observed sufficient expansion in the coarcted segment using a non-compliant balloon in an extremely low-birth-weight infant.
Instead of trying compliant balloons first, and then switching to the non-compliant balloon, we recommend the use of non-compliant balloons first with single lumen, high-pressure capability, and controlled expansion, which can pass through 4 F sheath in babies under 2000 g. They are more effective in the treatment of strick stenosis than compliant balloons and reduce the risk of vascular injury by decreasing the time spent using compliant balloons, which are likely to be ineffective in the vessel.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare no conflict of interests.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (please name) and with the Helsinki Declaration of 1975, as revised in 2008.





