An atrial shunt, or interatrial communication, is any opening in the atrial septum that allows blood to flow between the right and left atria.Reference Porter 1 Without early detection and appropriate treatment, a patient with an atrial shunt is at risk of developing pulmonary hypertension, heart rhythm abnormalities, right-sided heart failure, and paradoxical stroke.Reference English, Anderson and Ettedgui 2 – Reference Webb and Gatzoulis 4 Management may include monitoring, percutaneous closure, or surgical closure, and depends on the size and location of the defect, as well as the presence of associated defects. Therefore, diagnostic imaging is required to evaluate the shunt and to rule out associated defects such as partial anomalous pulmonary venous anatomy.Reference English, Anderson and Ettedgui 2 Currently, most patients clinically suspected of having an atrial shunt are referred for evaluation by trans-oesophageal echocardiography.Reference Webb and Gatzoulis 4 Recently, advances in cardiovascular magnetic resonance imaging have produced several different techniques that can be applied in the detection and quantification of atrial shunts, offering an alternative, non-invasive imaging modality.Reference Piaw, Kiam and Rapaee 5
This paper reviews the anatomy and presentation of atrial shunts in adults and children, and provides an overview of recommended clinical assessment including echocardiography, computed tomography, magnetic resonance imaging, and cardiac catheterisation. Focus will be placed on recent developments in magnetic resonance imaging that may improve the evaluation of atrial shunts and increase patient comfort.
Atrial septation and types of atrial shunts
In the foetus, elevated right atrial pressure and interatrial communication are essential to allow a bypass of the pulmonary circulation.Reference Whitaker 6 Atrial septation begins in the 6th week of development with infolding of the atrial roof to form a primary septum, led by a cap of mesenchymal cells, which migrate towards the endocardial cushions. The space between the primary septum and the endocardial cushions is the primary foramen.Reference Anderson, Brown and Webb 7 The spina vestibula, the ridge to the right of the entrance of a single, primitive pulmonary vein, also grows upward to connect the primary septum with the endocardial cushions. Before complete fusion with the endocardial cushions, the septum primum undergoes apoptosis superiorly to form the foramen ovale. At the 12th week of development, a second infolding from the atrial roof, septum secundum, occurs to the right of the primary septum, and descends just past the upper edge of the primary septum.Reference Anderson, Brown and Webb 7 This creates a one-way valve, allowing maintenance of the right-to-left shunt.
With postnatal respiration, a fall in pulmonary vascular resistance allows for increased pulmonary venous return, increasing the pressure in the left atrium relative to the right atrium so that the flap valve (upper edge of septum primum) closes against the septum secundum.Reference Anderson, Brown and Webb 7 With time, fibrous tissue permanently seals the septal structures together.Reference Porter 1 A patent foramen ovale results if the two septal membranes overlap but are not adherent, allowing a transient right-to-left shunt when right atrial pressure exceeds that of the left atrium.Reference Porter 1 , Reference English, Anderson and Ettedgui 2 According to an autopsy series by Hagen et al,Reference Hagen, Scholz and Edwards 8 a patent foramen ovale occurs in 25–30% of the population.
Distinct openings or holes allowing communication between the two atria are commonly known as atrial septal defects; however, it can be argued that not all are located within the true atrial septum. Recognising these distinctions as discussed below, we will use the terms atrial shunts, interatrial communication, and atrial septal defect throughout this review.Reference English, Anderson and Ettedgui 2 Atrial septal defects are recognised as the second most common congenital heart defect, with a reported incidence of 941 per million livebirths.Reference Hoffman and Kaplan 9 The true incidence is likely much higher, however, because many are diagnosed later in life.Reference English, Anderson and Ettedgui 2 Four types of atrial shunts other than a patent foramen ovale have been described based on their location relative to other cardiac structures.Reference Porter 1 , Reference Ho, Rigby and Anderson 10 These are illustrated in Figure 1.
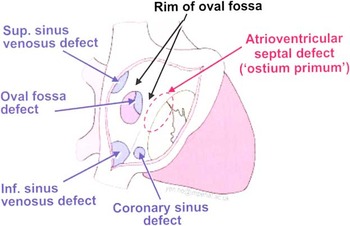
Figure 1 Illustration of the various types of interatrial communications. The oval fossa defect referred to here is commonly known as a secundum atrial septal defect. (Reproduced with permission of Imperial College Press, publisher of Echocardiography in Congenital Heart Disease Made Simple).Reference Ho, Rigby and Anderson 10
The secundum atrial septal defect is the most common, accounting for 70% of all atrial shunts.Reference Vogel, Berger, Kramer, Alexi-Meshkishvili and Lange 11 This defect occurs in the region of the oval fossa and results from the septum primum – the valve of the oval fossa – being absent, perforated, or too small to overlap the superior rim of the oval fossa, leaving the ostium secundum unguarded.Reference Porter 1 Roughly one quarter of patients with a secundum atrial septum defect also have a myxomatous abnormality of the mitral valve, which often results in mitral regurgitation.Reference Joy, Kartha and Balakrishnan 12
Ostium primum defects comprise 20% of atrial septal defects, and are located anterior to the fossa ovalis in the inferior part of the atrial septum, adjacent to the mitral and tricuspid valves.Reference Hoey, Gopalan, Ganesh, Agrawal and Screaton 13 These defects are often significant in size and result from persistence of the ostium primum. They are associated with abnormalities of the atrioventricular junction and valve, and hence are classified as atrioventricular septal defects.Reference Webb and Gatzoulis 4 , Reference Hoey, Gopalan, Ganesh, Agrawal and Screaton 13 Evaluation and treatment of ostium primum defects will not be further discussed in this review.
The less common sinus venosus defect – 2–3% of all atrial shunts – embryologically arises from a defect between the left atrium and the superior, or rarely the inferior, caval vein.Reference Veldtman, Freedom and Benson 3 Up to 85% of this type of defect are associated with partial anomalous pulmonary venous connections, where one or more of the pulmonary veins drain into the right atrium or the superior caval vein, rather than into the left atrium.Reference Webb and Gatzoulis 4 It is important to differentiate these defects from other atrial shunts as the majority of sinus venosus defects are not amenable to device closure and require surgical repair.
The most rare type of atrial shunt, a coronary sinus defect, occurs when an unroofed coronary sinus forms a shunt from the left atrium through the coronary sinus to the right atrium.Reference Webb and Gatzoulis 4 These are often associated with other defects such as left atrial connection of a persistent left superior caval vein, or, less commonly, pulmonary atresia.Reference Ho, Rigby and Anderson 10
Pathophysiology and natural history of atrial shunts
Although interatrial communication is essential to normal foetal circulation, it does alter the path of blood flow required for postnatal physiology by allowing shunting between the normally separated pulmonary and systemic circulations. In both the normal foetal and the pathologic postnatal circumstance, the direction and magnitude of flow through the atrial septal defect is dependent on the pressure gradient between the right and left atria, and the area of the defect.Reference Vick 14 In turn, the pressure gradient between the atria is determined primarily by the compliance of each atrium and its associated ventricle.Reference Vick 14 Beyond the first few weeks of life, normal mean right atrial pressure ranges from 0 to 6 mmHg,Reference Bustin 15 whereas left atrial pressure ranges from 4 to 12 mm of mercury.Reference Bustin 15 Therefore, in any isolated atrial septal defect, a primary left-to-right shunt will develop. This leads to a portion of the pulmonary venous return being redirected back through the lungs, and thus an increase in flow in the pulmonary circulation with a correlated decrease in systemic flow.Reference English, Anderson and Ettedgui 2 The ratio of pulmonary to systemic flow is a reflection of the magnitude and direction of blood flow through the defect; values of 1.5:1 or greater have been used to define a haemodynamically significant atrial shunt.Reference Sanders, Yeager and Williams 16 Although shunts identified in adults are likely to remain patent, in young children it is common for an atrial septal defect to close spontaneously.Reference Veldtman, Freedom and Benson 3
Secondary to the left-to-right shunt, diastolic stress from increased right ventricular volume stimulates the ventricle to synthesise new myocardial fibres in series, which increases the end-diastolic volume and impairs function.Reference Colan 17 Further, the increased chamber size contributes to systolic stress, which stimulates the heart to produce new fibres in parallel, thus contributing to increased wall thickness and excessive right ventricular trabeculations.Reference Colan 17 Over time, dilation of the right atrium, right ventricle, the pulmonary artery, and its branches may result.Reference Veldtman, Freedom and Benson 3 The tricuspid and pulmonary annuli may also become dilated and thickened, impairing their function.Reference Porter 1 Furthermore, ventricular interactions may occur, such that patients with right ventricular volume overload may also suffer from further decreased left ventricular output.Reference Colan 17 This phenomenon may be observed with cardiac imaging as diastolic septal flattening. Colan et alReference Colan 17 have described immediate normalisation of left ventricular function following transcatheter closure of an atrial septal defect.
Atrial stretch is hypothesised to play a role in the development of arrhythmias. Dilation of the right atrium has been shown to predispose patients to atrial fibrillation and atrial flutter,Reference Webb and Gatzoulis 4 which often produce initial symptoms such as palpitations, leading to cardiac investigation.Reference Gatzoulis, Freeman, Siu, Webb and Harris 18 Up to 45% of adults with unrepaired atrial septal defects have also been reported to have paroxysmal supraventricular tachycardia.Reference Meijboom, Hess and Szatmari 19
Chronic volume overload of the pulmonary circulation can, over time, lead to pulmonary hypertension, defined as a mean pulmonary arterial pressure >25 mmHg at rest and 30 mmHg with exercise.Reference Haworth and Rabinovitch 20 This may progress to an irreversible increase in pulmonary vascular resistance, characterised by intimal proliferation and fibrosis, leading to right ventricular failure, exercise intolerance, reduced quality of life, and death.Reference Haworth and Rabinovitch 20 A 1968 study of the natural history of 128 patients with a secundum atrial defect reported development of pulmonary vascular disease to be the most serious risk.Reference Craig and Selzer 21 A more recent studyReference Vogel, Berger, Kramer, Alexi-Meshkishvili and Lange 11 reported that at the time of presentation with a secundum atrial septal defect, ∼9% of patients have already developed pulmonary arterial hypertension. Cherian et alReference Cherian, Uthaman and Durairaj 22 reported pulmonary hypertension in 13% of patients under 10 years of age, and in 14% of patients in the age group of 11–20 years. Pulmonary arterial hypertension is three times more prevalent in patients with sinus venosus defects compared with those with secundum defects,Reference Vogel, Berger, Kramer, Alexi-Meshkishvili and Lange 11 as most of these patients have additional shunting through partial anomalous pulmonary venous connections. Vogel et alReference Vogel, Berger, Kramer, Alexi-Meshkishvili and Lange 11 observed that pulmonary disease develops at a younger age in these patients, warranting additional monitoring and earlier treatment.
In the presence of pulmonary hypertension, elevated right ventricular pressure causes movement of the interventricular septum towards the left. This can be visualised as systolic septal flattening so that both ventricles appear D-shaped on a short-axis image. This process may further limit left ventricular filling.Reference Porter 1 Pulmonary pressures can eventually rise above systemic pressures, leading to reversal of the shunt.Reference Veldtman, Freedom and Benson 3 Known as Eisenmenger syndrome, this condition is characterised by cyanosis with associated findings including clubbing, polycythaemia, and coagulopathy.Reference Veldtman, Freedom and Benson 3 Cherian et alReference Cherian, Uthaman and Durairaj 22 identified Eisenmenger syndrome in 9% of their 709 consecutive atrial septal defect patients, with little difference between younger and older patients. Although the right-to-left shunt is pathological, it is generally agreed that these shunts should not be closed in the presence of irreversible pulmonary hypertension, as they minimise progression of pulmonary disease and may help maintain systemic cardiac output.Reference Vick 14
Lastly, patients with atrial shunts may have a slightly increased risk for stroke because of the possibility of a transient right-to-left shunt allowing paradoxical embolism.Reference English, Anderson and Ettedgui 2 The need to repair the shunt or to anticoagulate these patients because of this risk remains controversial.
In summary, the course of patients with an unrepaired atrial shunt is extremely variable and is dependent on the age at presentation, the type and severity of the shunt, the ratio of pulmonary-to-systemic vascular resistance, and the compliance of the left and right ventricles.Reference Porter 1
Clinical presentation and initial evaluation
Symptoms
While nearly always present from birth, atrial shunts may be diagnosed at any age as the severity of symptoms, which are often non-specific, vary greatly with the significance and progression of disease.Reference Webb and Gatzoulis 4 Most infants with atrial septal defects are asymptomatic, although mild transient cyanosis can occur in the newborn as a result of a right-to-left shunt.Reference Vick 14 Children and adults with a significant shunt might complain of fatigue, dypsnoea, or exercise intolerance.Reference English, Anderson and Ettedgui 2 , Reference Webb and Gatzoulis 4 They may also experience palpitations because of atrial fibrillation or flutter, but this typically does not occur in patients before the age of 40.Reference Gatzoulis, Freeman, Siu, Webb and Harris 18
Clinical examination
The heart rate, blood pressure, and respiratory rate are typically normal in a patient with an atrial shunt.Reference English, Anderson and Ettedgui 2 Jugular venous pressure may be normal;Reference English, Anderson and Ettedgui 2 however, prominence of the “v” wave in comparison with the “a” wave is sometimes apparent and is considered a valuable clinical sign of an atrial septal defect.Reference Tavel and Stewart 23 , Reference Parikh, Fisher and Moses 24 Older children and adults with large shunts might have a precordial bulge, a right ventricular heave, and/or a prominent systolic impulse indicating an enlarged right ventricle.Reference Porter 1 , Reference English, Anderson and Ettedgui 2
The heart sounds of a patient with an atrial septal defect are rarely normal.Reference English, Anderson and Ettedgui 2 The first heart sound at the lower left sternal border is often accentuated. A fixed and widely split second heart sound has been described as the most valuable sign of a left-to-right interatrial shunt.Reference English, Anderson and Ettedgui 2 Closure of the pulmonary valve is delayed because of volume overload of the right heart, and the delay between aortic and pulmonary valve closure is fixed because the already volume loaded right heart is unable to further increase filling with inspiration. The murmur of an atrial septal defect is typically a soft crescendo–decrescendo systolic ejection murmur at the second left intercostal space reflecting increased volume passing through the pulmonary valve. There also might be a mid-diastolic murmur at the lower left sternal border, suggesting increased flow across the tricuspid valve.Reference Porter 1 , Reference English, Anderson and Ettedgui 2 , Reference Vick 14 Pulmonary hypertension, if it has developed, will result in a loud second pulmonary sound, elimination of the widely split second heart sound and the diastolic murmur, and a shorter systolic murmur.Reference Porter 1 , Reference Webb and Gatzoulis 4
Chest radiography
Chest radiography is not indicated if an atrial shunt is suspected; however, in most patients with a significant atrial shunt, a chest radiograph will demonstrate an enlarged right heart and a dilated pulmonary trunk.Reference English, Anderson and Ettedgui 2 Increased pulmonary vascular markings suggest increased pulmonary blood flow, but decreased vascularity may be seen in the presence of pulmonary vascular disease.Reference Rees, Farru and Rodriguez 25
Electrocardiogram
Patients with an atrial shunt typically have a normal heart rate with normal sinus rhythm; however, in patients older than 40 years, atrial fibrillation or flutter is common.Reference English, Anderson and Ettedgui 2 An electrocardiogram may be useful in identifying the type of atrial shunt. For example, a leftward or left superior QRS axis suggests an ostium primum defect.Reference Webb and Gatzoulis 4 With most secundum defects, the axis will be normal or rightward (90–170°).Reference English, Anderson and Ettedgui 2 Inverted P waves in the inferior leads may indicate a sinus venosus defect.Reference Webb and Gatzoulis 4 Electrocardiography is also useful in detecting associated anomalies such as right ventricular hypertrophy, indicated by RSR′ pattern in the right precordial leads.Reference English, Anderson and Ettedgui 2 , Reference Webb and Gatzoulis 4 , Reference Cohen, Patton and Giuffre 26 First-degree atrioventricular block is not uncommon in atrial septal defect patients, and a right bundle branch block is present in half of the patients over 60 years of age.Reference English, Anderson and Ettedgui 2
Exercise testing
Many patients with atrial septal defects have reduced cardiopulmonary exercise capacity.Reference Giardini, Donti and Formigari 27 , Reference Suchon, Podolec and Tomkiewicz-Pajak 28 Assessment of a patient’s maximal oxygen uptake (VO2 max) and “oxygen pulse” – amount of oxygen consumed per heartbeat – compared with normal age- and sex-specific values contributes additional information for select patients in whom additional assessment of the haemodynamic significance of the shunt is required, or in following improvement of symptoms post repair.Reference Giardini, Donti and Formigari 27 – Reference Radojevic and Rigby 29
Advanced investigations and imaging
Before recommending treatment, diagnostic imaging is required to confirm the diagnosis, and to determine the shunt location, size, and haemodynamic significance, and to rule out associated anomalies.Reference English, Anderson and Ettedgui 2 According to European Society of Cardiology guidelines,Reference Baumgartner, Bonhoeffer and De Groot 30 diagnostic work-up generally includes transthoracic echocardiography as initial screening, and trans-oesophogeal echocardiography to complete assessment. If this is insufficient to determine a management plan, then cardiovascular magnetic resonance imaging, computed tomography, and/or cardiac catheterisation may be required.
Echocardiography
Echocardiography has become the primary imaging technique for the evaluation of atrial septal defects. Right ventricular size and function can be assessed, and pulmonary arterial pressure and shunt fraction can also be estimated with transthoracic echocardiography using Doppler imaging. In children, the subcostal four-chamber view has been reported as the most diagnostic of atrial shunts (Fig 2a and b).Reference Ho, Rigby and Anderson 10 Unfortunately, in adults this view is typically too far from the heart and is not useful. Overall, the limited acoustic windows of transthoracic echocardiography do not allow complete visualisation of the atrial septum or other posterior cardiac structures, and further assessment is typically required in many older children and most adults before recommending treatment.Reference Gnanapragasam, Houston, Northridge, Jamieson and Pollock 31 , Reference Fischer, Stieh, Uebing, Hoffmann, Morf and Kramer 32

Figure 2 Echocardiographic images of atrial shunts. (a, b) Transthoracic subcostal view of a 9-day-old infant with a large secundum atrial septal defect (arrow) with left-to-right shunt. (c) Trans-oesophageal echocardiography demonstrating a large atrial shunt (arrow) in a 28-year-old female. LA=left atrium; LV=left ventricle; RA=right atrium.
Trans-oesophageal echocardiography is currently the modality of choice to evaluate an atrial shunt, as it provides an excellent view of the interatrial septum (Fig 2c) and often demonstrates pulmonary venous anatomy.Reference Gnanapragasam, Houston, Northridge, Jamieson and Pollock 31 , Reference Fischer, Stieh, Uebing, Hoffmann, Morf and Kramer 32 It is considered important for characterisation of an atrial shunt and for determining the best course of treatment, as well as to guide percutaneous closure of secundum defects.Reference Lerakis and Babaliaros 33 In addition to assessment of the diameter of the defect, multi-planar trans-oesophageal echocardiography is used to assess the rims of septum surrounding the defect. The superior and inferior vena caval rims are imaged in the bicaval view, the atrioventricular valve rim in a four-chamber view, and retro-aortic rim in a short-axis plane.Reference Lerakis and Babaliaros 33 A deficient (<5 mm) rim to the atrioventricular valve, superior or inferior vena cava, or right upper pulmonary vein typically precludes device closure, whereas a deficient retro-aortic rim in multiple sequential views requires careful consideration of the choice of septal occlusion device versus surgical repair.Reference Lerakis and Babaliaros 33 Visualisation of all pulmonary veins and any sinus venosus defects remains especially challenging.Reference Hoey, Gopalan, Ganesh, Agrawal and Screaton 13 Furthermore, the procedure can be uncomfortable for the patient, is associated with a small risk of oesophageal trauma, and requires sedation and a topical anesthetic, or general anesthesia in children.Reference Piaw, Kiam and Rapaee 5
Saric et alReference Saric, Perk, Purgess and Kronzon 34 have demonstrated that three-dimensional trans-oesophageal echocardiography acquires accurate views of the atrial septum, with an additional advantage that it can provide an “en face” (in plane) view of the atrial septum. Signal dropout within the atrial septum often occurs in normal patients with three-dimensional trans-oesophageal imaging, decreasing the specificity of this technique. The inferior rim of the IVC is also not easily visualised in many patients.Reference Saric, Perk, Purgess and Kronzon 34 Despite these limitations, three-dimensional trans-oesophageal echocardiography has also been shown to successfully guide percutaneous closure of atrial shunts.Reference Lodato, Cao and Weinert 35
Intracardiac echocardiography has also been utilised in some centres for imaging atrial shunts.Reference Durongpisitkul, Tang, Soongswang, Laohaprasitiporn and Nanal 36 It is not yet recognised as a routine diagnostic tool, but has proven useful in guiding transcatheter closure,Reference Durongpisitkul, Tang, Soongswang, Laohaprasitiporn and Nanal 36 , Reference Zanchetta, Rigatelli, Pedon, Zennaro, Carrozza and Onorato 37 and was reported to be more comfortable for the patient than trans-oesophageal echocardiography.Reference Hernandez, Garcia-Tejada, Velazquez, Albarran, Andreu and Tascon 38 In the axial plane of the aortic valve, the atrial septum is well seen and, according to Onorato et al,Reference Onorato, Casilli and Zanchetta 39 the dimensions and rims of an atrial septal defect may be measured accurately. A view in the axial plane of the junction between the superior caval vein and the right atrium provides easy identification of any anomalous pulmonary veins.Reference Onorato, Casilli and Zanchetta 39
Catheterisation and angiography
Cardiac catheterisation has historically been recognised as among the most accurate methods of quantifying shunts and measuring pressure.Reference English, Anderson and Ettedgui 2 However, because of its invasive nature, this technique is now reserved for immediately before percutaneous closure.Reference Hundley, Li and Lange 40 If the pulmonary arterial pressure as estimated by echocardiography is >50% of the systemic pressure, catheterisation is indicated to assess for pulmonary hypertension.Reference Levin, Spach, Boineau, Canent, Capp and Jewett 41
While mean right atrial pressure is typically normal in the presence of an atrial septal defect, the “v” and “a” waves are often equal.Reference English, Anderson and Ettedgui 2 , Reference Parikh, Fisher and Moses 24 Right ventricular and pulmonary artery pressures are also usually normal unless the defect is very large. Pulmonary arterial pressures have been observed to increase with age and shunt severity,Reference English, Anderson and Ettedgui 2 and the gradient across the pulmonary valve is increased in most atrial septal defect patients.Reference Vick 14 The pressure gradient between the right and left atrium is usually small, particularly in large shunts.Reference Vick 14 , Reference Levin, Spach, Boineau, Canent, Capp and Jewett 41
Invasive oximetry measures blood oxygen saturation in various cardiac chambers. An atrial shunt is considered present when the oxygen saturation in the right atrium is at least 5% higher than that in the superior caval vein in the absence of other anomalies.Reference English, Anderson and Ettedgui 2 Applying the Fick principle allows for estimation of the shunt ratio (pulmonary flow: aortic flow). Pulmonary flow can be calculated as the oxygen consumption (ml/min) divided by the arteriovenous oxygen content difference across the lungs (ml/L), whereas aortic flow is equal to oxygen consumption (ml/min) divided by the arteriovenous oxygen content difference across the body (ml/L).Reference Boehrer, Lange, Willard, Grayburn and Hillis 42
Being among the oldest techniques applied in cardiac shunt detection, invasive oximetry is often used as a standard to validate newer shunt quantification techniques. However, Boehrer et alReference Boehrer, Lange, Willard, Grayburn and Hillis 42 argue that oximetry is unable to detect small interatrial shunts and provides only an estimate of shunt fraction.
Although rarely used now, the indicator dilution technique is of historical interest. It involves intravenous injection of a bolus of indicator dye, most often indocyanine green, and withdrawal of blood from the arterial circulation in order to measure the indicator concentration. Concentration of the indicator is plotted against time, and demonstrates a large primary peak followed by a normal recirculation peak in patients without a left-to-right shunt. If a left-to-right shunt is present, the primary peak will be of lesser amplitude, and a prominent early recirculation peak will be present because of blood with indicator bypassing the pulmonary circulation to return directly to the systemic circulation.Reference Rees, Farru and Rodriguez 25 , Reference Hundley, Li and Lange 40 , Reference Boehrer, Lange, Willard, Grayburn and Hillis 42 This technique is useful for calculating a pulmonary-to-systemic flow ratio, and has been shown to correlate well with values obtained from oximetry,Reference Jarmakani 43 although oximetry provides slightly higher shunt ratio values than does the indicator dilution technique.Reference Rees, Farru and Rodriguez 25 Its sensitivity in detecting small shunts is slightly better than oximetry, detecting a shunt ratio as small as 1.35; however, it fails to provide any information on shunt location.Reference Boehrer, Lange, Willard, Grayburn and Hillis 42
According to Boehrer et al,Reference Boehrer, Lange, Willard, Grayburn and Hillis 42 contrast angiography is useful for detecting cardiac shunts such as ventricular septal defects, but is often insensitive to detection and quantification of atrial shunts. Nevertheless, it can be useful for depiction of pulmonary artery and pulmonary vein anatomy, as imaged from the antero-posterior or lateral views.Reference English, Anderson and Ettedgui 2
X-Ray computed tomography
In general, cardiac computed tomography is known to provide information complementary to echocardiography or magnetic resonance imaging. Its excellent spatial resolution makes it particularly useful in detecting associated anomalies and assessing changes in the pulmonary vasculature.Reference Hoey, Gopalan, Ganesh, Agrawal and Screaton 13 Williamson et alReference Williamson, Kirsch and Araoz 44 recently applied an electrocardiography-gated cardiac computed tomography angiography technique with a saline chaser to 20 patients, and found that computed tomography has high sensitivity in detecting a patent foramen ovale based on the presence of an atrial septal flap, a continuous contrast column between the atria, and a jet of contrast into the right atrium.
Current applications of magnetic resonance imaging in atrial shunt assessment
Cardiovascular magnetic resonance imaging can provide a valuable assessment of cardiac anatomy, function, blood flow, and tissue characteristics.Reference Hundley and Bluemke 45 Furthermore, it is non-invasive and applies no ionising radiation.Reference Webb and Gatzoulis 4 Several cardiac magnetic resonance imaging techniques have been shown to be valuable in assessing atrial shunts.Reference Pennell, Sechtem and Higgins 46 , Reference Teo, Disney and Dundon 47
Steady-state free precession cine imaging
Steady-state free precession is a fast gradient echo pulse sequence used to acquire cine images during a single breath hold.Reference Lamb, Kozerke, Doornbos, Bax and de Roos 48 This sequence is excellent for determining diastolic and systolic ventricular volumes as shown in Figure 3, and for calculating ejection fraction; therefore, it is a fundamental sequence used in nearly every cardiac magnetic resonance imaging protocol.Reference Sandner, Theisen, Bauner, Picciolo, Reiser and Wintersperger 49 If an intracardiac shunt is present, it can be quantified by calculating the biventricular stroke volume ratio, which should be equal to the ratio of pulmonary arterial flow to aortic flow (Qp/Qs) in a patient with no other cardiac shunt. The net shunt flow may be calculated as the difference between the left and right ventricular stroke volumes.Reference Colletti 50

Figure 3 One slice of a stack of ventricular short-axis steady-state free precession images in diastole (a) and systole (b) demonstrating contouring of the right ventricle in assessment of stroke volume.
In some cases, an interrupted septum may be seen in the four-chamber view and short-axis atrial stack cines (Fig 4), and flow may be visible across the defect.Reference Teo, Disney and Dundon 47 , Reference Hamilton-Craig, Sestito and Natale 51 A recently published study demonstrated cardiac magnetic resonance imaging to have excellent assessment of size, location, and septal rims compared with trans-oesophageal echocardiography in 20 patients using solely cardiac-gated steady-state free precession sequences.Reference Teo, Disney and Dundon 47 The maximum and minimum size of the defect measured by steady-state free precession correlated well with trans-oesopheal echocardiography (r=0.87 and 0.92), but had a weaker correlation with size of the device used in percutaneous closure (r=0.53 and 0.57 for maximum and minimum size, respectively).Reference Teo, Disney and Dundon 47 Despite these results, experts maintain that steady-state free precession images have low specificity in atrial shunt detection, and do not provide accurate defect size.Reference Johnson and Fogel 52

Figure 4 Still frames of steady-state free precession MRI cines at 1.5 Tesla depicting an atrial septal defect in (a) a four-chamber view and (b) atrial short-axis view. An interrupted atrial septum and dephasing of blood is obvious in the four-chamber view, but not in the short-axis view. MRI=magnetic resonance imaging.
Gradient-recalled echo cines with saturation band
In this technique, a pre-saturation radiofrequency pulse is applied before each image acquisition, which produces saturation of spins – preventing any signal – in a specific area or band.Reference Johnson and Fogel 52 For assessment of an atrial shunt, the saturation band is placed on either the left or the right atrium, parallel with the atrial septum. Flow through a shunt is clearly visualised by the movement of dark or bright blood into the opposing atrium, as seen in Figure 5.Reference Johnson and Fogel 52 The dimensions of the defect can be measured in both the four-chamber and atrial short-axis views to provide an estimate of area. Saturation bands could potentially be more sensitive than trans-oesophageal echocardiography in detecting unusually located atrial shunts.
First-pass gadolinium perfusion
Originally used for detection of coronary artery disease, first-pass gadolinium perfusion has been applied to dynamic imaging of atrial shunts.Reference Hamilton-Craig, Sestito and Natale 51 A bolus of gadolinium contrast is injected intravenously and the atria and pulmonary veins are imaged rapidly using a fast gradient echo sequence. In the presence of an atrial septal defect with left-to-right shunting, dark blood can be seen entering the right atrium when it is filled with contrast, and bright blood is seen re-circulating into the right atrium from the contrast-filled left atrium (Fig 6). If contrast appears in the left atrium after the right atrium, but before the pulmonary veins, then a right-to-left shunt is present.Reference Hamilton-Craig, Sestito and Natale 51 Compared with trans-oesophageal echocardiography in Hamilton-Craig’s study, first-pass perfusion is less sensitive in the detection of a patent foramen ovale and tends to underestimate the size of the shunt.Reference Hamilton-Craig, Sestito and Natale 51

Figure 5 Gradient-recalled echo with saturation band method shows a small shunt in four-chamber (a) and atrial short-axis (b) views.
Phase contrast velocity
This sequence makes use of motion-induced phase shift to quantify velocity and flow either through-plane or in-plane.Reference Thomson, Crowley and Heitner 53 In atrial shunts, velocity and flow are routinely quantified through the proximal ascending aorta and the main pulmonary artery in order to calculate the shunt ratio (Qp/Qs).Reference Hoey, Gopalan, Ganesh, Agrawal and Screaton 13 , Reference Hundley, Li and Lange 40 , Reference Beerbaum, Korperich, Barth, Esdorn, Gieseke and Meyer 54 Accuracy is highly dependent on technical expertise: phase contrast velocity requires careful planning to position the imaging plane, select appropriate spatial and temporal resolution, and post-processing also requires attention to detail. Turbulence, partial volume effects, and phase-offset or background errors can contribute to inaccuracies,Reference Fratz, Chung and Greil 55 some of which can be corrected with the use of a phantom, or by measuring the phase-offset error in a region of no flow near the area of interest, typically the chest wall.Reference Gatehouse, Rolf and Graves 56 Aortic and main pulmonary artery flow assessment may have additional errors because of through-plane motion of the annulus, impact of vessel compliance, and complex flow at the coronary ostia.Reference Fratz, Chung and Greil 55 Internal validation is possible by verifying that flow through the main pulmonary artery is equal to the either the sum of the flow through the left and right pulmonary arteries or that pulmonary arterial flow is equal to pulmonary venous return.Reference Hundley, Li and Lange 40 , Reference Colletti 50 , Reference Fratz, Chung and Greil 55 Beerbaum et alReference Beerbaum, Korperich, Barth, Esdorn, Gieseke and Meyer 54 , Reference Beerbaum, Parish, Bell, Gieseke, Korperich and Sarikouch 57 demonstrated that shunt ratio determination by phase contrast velocity in children is as reliable as invasive oximetry. Figure 7 demonstrates a phase contrast and a magnitude image of the aorta in through-plane view.

Figure 6 Still frames of perfusion imaging clearly demonstrate a large left-to-right shunt. (a) Blood without contrast from the left atrium is seen entering the right atrium, which is filled with contrast. (b) When the contrast has entered the left atrium, it is seen recirculating into the right atrium.
Phase contrast velocity can also be applied to measure flow directly through an atrial septal defect,Reference Beerbaum, Korperich and Esdorn 58 as shown in Figure 8. Thomson et alReference Thomson, Crowley and Heitner 53 recently investigated the accuracy of this method in flow quantification of secundum defects. The atrial shunt ratio was calculated in three ways: the sum of systemic and shunt flow divided by systemic flow; pulmonary flow divided by the sum of pulmonary and shunt flow; and the pulmonary-to-systemic flow ratio. They found the first shunt ratio to correlate best with oximetry results (r=0.89), compared with the latter calculations (r=0.77 and 0.74, respectively), suggesting that direct en face imaging of atrial shunts may provide a more accurate hemodynamic assessment.Reference Thomson, Crowley and Heitner 53

Figure 7 Phase contrast velocity (a) and magnitude image (b) of the aorta in short-axis or through-plane view (arrow).
En face flow also demonstrates the shape and size of an atrial septal defect. Phase contrast cine images have been shown to be more accurate in evaluating the size and shape of atrial septal defects than both trans-oesophageal and intracardiac echocardiography.Reference Piaw, Kiam and Rapaee 5 , Reference Thomson, Crowley and Heitner 53 , Reference Holmvang, Palacios and Vlahakes 59 In a study of 30 patients with atrial sepal defects, Holmvang et alReference Holmvang, Palacios and Vlahakes 59 observed that phase-contrast cine images were adequate to define the defect’s shape and size. The maximum diameter by phase contrast magnetic resonance imaging correlated closely with balloon sizing measurements (r=0.75) and with templates cut during surgery (r=0.93).Reference Holmvang, Palacios and Vlahakes 59 In 32 patients who underwent Amplatzer device closure, Thomson et alReference Thomson, Crowley and Heitner 53 found that the area of the atrial shunt measured by phase contrast velocity correlated with the size of the deployed Amplatzer device better than intracardiac echocardiography, especially in patients who received small- to medium-sized devices – area <3 cm2. According to Colletti et alReference Colletti 50 this is the best method to determine the size and directionality of intracardiac shunts. A study of 54 children who underwent catheter closure with the Amplatzer septal occluder demonstrated that cardiac magnetic resonance imaging was more accurate at calculating atrial septal defect diameter relative to balloon sizing in comparison to trans-oesophageal echocardiography.Reference Durongpisitkul, Tang, Soongswang, Laohaprasitiporn and Nanal 36 Finally, rim measurements can be measured with this technique, which is essential in determining whether percutaneous closure is possible.Reference Johnson and Fogel 52 , Reference Beerbaum, Korperich and Esdorn 58 Rim assessment using this technique is limited outside of major centres; therefore, trans-oesophageal echocardiography remains the gold standard for rim assessment.
Four-dimensional flow is a three-dimensional phase contrast velocity sequence that acquires the blood flow patterns in the entire thoracic cavity in a single acquisition, and offers particle tracing. This allows for quantification of flow through the aorta, pulmonary artery, and atrial septal defect with simplified planning, reducing overall time required and potentially increasing accuracy.Reference Valverde, Simpson, Schaeffter and Beerbaum 60 Valverde et alReference Valverde, Simpson, Schaeffter and Beerbaum 60 highlighted these advantages in an 8 year old with a secundum atrial septal defect and anomalous pulmonary veins, which were not seen on transthoracic echocardiography. At this time, four-dimensional flow is a promising sequence, but is time-consuming and not used in most protocols for assessment of atrial shunts.
Magnetic resonance angiography
One of the main advantages of cardiac magnetic resonance imaging over other imaging modalities is its excellent delineation of anatomy of the great arteries and pulmonary veins,Reference Wang, Reddy, Gotway, Yeh and Higgins 61 as seen in Figure 9, using gadolinium contrast angiography, without exposing the patient to ionising radiation.Reference Johnson and Fogel 52 This is important for detection of anomalous pulmonary venous connections, which, if present, may preclude device closure.Reference Prasad, Soukias and Hornung 62

Figure 8 Phase contrast velocity image of an atrial septal defect en face. The area was 179 mm2.
Current time, cost, and limitations
Having multiple sequences that can acquire the same information is a major strength of cardiac magnetic resonance imaging, providing internal validation, unlike other imaging modalities.Reference Fogel 63 The time required to complete all of the above sequences applied in atrial shunt evaluation exceeds 1 hour, although imaging time may be reduced with judicious selection of sequences. Of note, gadolinium contrast, required for first-pass perfusion and magnetic resonance imaging angiography, is contraindicated in patients with renal failure because of an association with nephrogenic systemic fibrosis, a rare but devastating and often fatal disease.Reference Juluru, Vogel-Claussen, Macura, Kamel, Steever and Bluemke 64 Contrast is also contraindicated in patients who are pregnant or breastfeeding. Magnetic resonance imaging should not be performed in pregnant women,Reference Ain, Narula and Sengupta 65 patients with intracardiac electronic devices,Reference Marinskis, Bongiorni and Dagres 66 or extreme claustrophobia unless the benefits are deemed to outweigh the risks.
Treatment
Progression of symptoms and adverse pathophysiology can be prevented with closure of the interatrial communication, either by open-heart surgery or percutaneously with the use of a device.Reference Therrien, Warnes and Daliento 67 Before recommending a treatment, a cardiologist will need to consider the patient’s age and symptoms, the shunt ratio – pulmonary artery to aortic flow – the size, shape, and location of the defect, the adequacy of the septal rims, the proximity of the defect to other cardiac structures, the pulmonary vascular resistance, and associated defects.Reference Veldtman, Freedom and Benson 3
Repair of an atrial shunt is indicated if the shunt ratio is >1.5:1, or if the defect is at least 10 mm in diameter in adults, regardless of the symptoms of the patient.Reference Webb and Gatzoulis 4 , Reference Therrien, Warnes and Daliento 67 , Reference Daniel, Lange, Willard, Landau and Hillis 68 One exception to this rule is if the patient is <2 years of age, as there is a high probability of spontaneous closure.Reference English, Anderson and Ettedgui 2 As previously indicated, these values have been recognised as causative of right heart dilation, increased pulmonary pressures, and other adverse outcomes of a chronic left-to-right shunt.Reference Therrien, Warnes and Daliento 67 Repair is generally not recommended in patients with small atrial shunts; however, these patients should be monitored for development of right heart dilation or symptoms, and then closure should be considered.Reference English, Anderson and Ettedgui 2 , Reference Webb and Gatzoulis 4
Repair of an atrial shunt is contraindicated if pulmonary hypertension has already developed (greater than eight Woods units), or if right ventricular function is severely compromised.Reference Fu, Cao and Hijazi 69 In these cases, closing the shunt is likely to worsen the patient’s clinical outcome. Closure in pregnant women is typically delayed until 6 months following delivery.Reference Webb and Gatzoulis 4
Anticipated benefits from atrial septal defect closure include improved exercise capacity and quality of life, prevention of pulmonary hypertension and right heart failure, and reduced risk of stroke and arrhythmias.Reference Therrien, Warnes and Daliento 67 Remodelling of the right ventricle and right atrium to normal size has been demonstrated in treated patients. However, remodelling is often incomplete in older patients, with 26–29% of patients having persisting right ventricular dilation 1 year after atrial septal defect repair.Reference Meijboom, Hess and Szatmari 19 The prevalence of atrial fibrillation is reduced after surgical repair, especially in younger patients. Of 192 patients who had surgical repair of atrial shunt, the age of those with persistent atrial fibrillation was 47.7±19 years compared with 22.9 years for resolved atrial fibrillation.Reference Oliver, Gallego and Gonzalez 70
Percutaneous closure
Transcatheter closure of an atrial septal defect was first performed in 1976.Reference King, Thompson, Steiner and Mills 71 The initial devices were not easy to place accurately within the defect and some had a high fracture rate.Reference English, Anderson and Ettedgui 2 Since the development of new occluders such as the Amplatzer device (Fig 10), percutaneous closure of atrial septal defects has significantly increased. Device closure is now as effective as surgery and the primary method of choice. Benefits include no thoracotomy, a shorter stay in hospital, less pain and discomfort to the patient, and lower cost.Reference English, Anderson and Ettedgui 2

Figure 9 Thick maximum intensity projection of pulmonary vein angiogram, transverse orientation (a). Volume-rendered display of pulmonary vein angiogram, posterior orientation (b).

Figure 10 Photographs of an Amplatzer Atrial Septal Occluder device. (a) View from the side demonstrating two discs connected by a thinner waist. (b) View of the device from the front demonstrating its diameter.
The primary contraindications to percutaenous closure of the atrial shunt include: too large a defect – diameter >36 mm, or if the device required is too large for the patient – inadequate septal tissue,Reference Lerakis and Babaliaros 33 or if the defect is too close to other cardiac structures, such as the atrioventricular valves, upper pulmonary veins, or the coronary sinus. In addition, percutaneous closure is not recommended if the femoral veins are too small to allow access, or if the patient has intracardiac thrombi, other cardiac defects, bleeding disorders, any infection, or a contraindication to aspirin or other anticoagulants.Reference Webb and Gatzoulis 4
The procedure is performed with the patient under general anaesthesia or local anaesthesia with sedation.Reference English, Anderson and Ettedgui 2 A catheter is guided into the right heartReference Fu, Cao and Hijazi 69 where pulmonary artery pressure and pulmonary vascular resistance are determined. A sizing balloon is used to acquire the “balloon size”, defined as the diameter of the balloon as measured by echocardiography when complete obstruction of Doppler flow is achieved.Reference Lerakis and Babaliaros 33 , Reference Fu, Cao and Hijazi 69 Next, a delivery sheath for the device is advanced into the left atrium and the occluding device is passed through the sheath.Reference Gervasi and Basu 72 The Amplatzer septal occluder, which consists of two attached discs made of a polyester fabric encased by a woven wire mesh of nickel-titanium, is then extruded from the catheter, so that a disc resides on either side of the septum.Reference English, Anderson and Ettedgui 2 , Reference Fu, Cao and Hijazi 69 , Reference Gervasi and Basu 72 , Reference Bjornstad 73 Alternatively, a GORE HELEX septal occluder (W.L. Gore and Associates, Flagstaff, Arizona, USA) may be used, which is a nickel-titanium wire covered with Gore-Tex.Reference Gervasi and Basu 72 , Reference Bjornstad 73 Appropriate positioning of the device can be verified using fluoroscopy, trans-oesophageal or intracardiac echocardiography, and angiography before deployment.Reference Fu, Cao and Hijazi 69
A known complication of percutaneous atrial shunt repair is erosion of the device through adjacent cardiac structures. To avoid this complication, it is important to assess the retro-aortic rim of the defect carefully, and to ensure the device is not significantly larger than the defect.Reference English, Anderson and Ettedgui 2 Other possible complications include embolisation of the device, tamponade from cardiac trauma, thrombosis, and arrhythmias.Reference English, Anderson and Ettedgui 2
Surgical closure
If transcatheter atrial shunt closure is contraindicated, or if the patient prefers surgical repair, it may be necessary to perform open-heart surgery.Reference English, Anderson and Ettedgui 2 Atrial septal defects are often closed directly with sutures, or with a patch of either pericardium or synthetic material. Associated defects, if present, are usually repaired at the same time.Reference Webb and Gatzoulis 4 This surgery has been performed since the 1950s and now has an excellent success rate and near-zero mortality.Reference English, Anderson and Ettedgui 2 , Reference Alexi-Meskishvili and Konstantinov 74
Post-operative complications are rare, but can include transient or chronic arrhythmias – sinus node dysfunction, supraventricular tachycardia – or post-pericardiotomy syndrome – an inflammatory reaction.Reference English, Anderson and Ettedgui 2 Obstruction of the superior vena cava or a right-sided anomalous vein is possible following repair of a sinus venosus defect.Reference English, Anderson and Ettedgui 2
Post-closure complications
After either method of atrial septal defect repair, ongoing concerns include mitral or tricuspid regurgitation, persistent right ventricular and atrial dilation – more commonly in older patients or those with increased pulmonary vascular resistance – and complications such as atrial fibrillation, thrombus, and sinus and/or atrioventricular node dysfunction.Reference Veldtman, Freedom and Benson 3 , Reference Gatzoulis, Freeman, Siu, Webb and Harris 18 , Reference Luermans, Post and Yilmaz 75 Rarely, Budd–Chiari syndrome – occlusion of the hepatic veins – may develop.Reference Veldtman, Freedom and Benson 3 Bacterial endocarditis is very rare, but cases have been reported following device closure – one at 8 weeks after the procedure and the other at 10–14 weeks after repair. Antibiotic prophylaxis is recommended for at least 6 months following repair, particularly in patients who underwent percutaneous repair.Reference Veldtman, Freedom and Benson 3
Summary and recommendations
Atrial shunts are a relatively common congenital heart defect that may lead to adverse effects such as right heart dilation, atrial arrhythmias, and pulmonary arterial hypertension. Symptoms including shortness of breath, reduced exercise tolerance, and palpitations may occur. Haemodynamically significant shunts can be repaired by percutaneous closure with an occluder, or by open-heart surgery, depending on the characteristics of the patient and of the atrial septal defect. Before a decision on treatment, a shunt must first be evaluated with advanced diagnostic imaging, and closure should be performed only after weighing the potential risks and benefits to the individual.
Transthoracic echocardiography is used as a first-line test, particularly in children, to diagnose an atrial shunt, and trans-oesophageal echocardiography is currently the primary imaging modality in its confirmation and assessment. Cardiac magnetic resonance imaging is currently used to provide additional information in unclear situations; however, evidence suggests that it approaches the sensitivity, specificity, and sizing accuracy of trans-oesophageal echocardiography with the added advantage of being non-invasive. Further research may refine the cardiac magnetic resonance imaging protocol to optimise evaluation of atrial shunts, applying only the most accurate and fastest sequences, so that it may replace trans-oesophageal echocardiography in the future.
Acknowledgements
The authors wish to acknowledge the Alberta Children’s Hospital Research Institute and the Stephenson Cardiovascular Magnetic Resonance Imaging Centre for their support of this work.












