Percutaneous closure of atrial septal defects has evolved as the treatment of choice for closure of the majority of such defects. Usually, the aim of the treatment is complete occlusion, but in some clinical scenarios complete closure is not desirable and the maintenance of a small communication is required to allow decompression of one or other atrium. As Galiè et alReference Galiè, Hoeper and Humbert 1 describe, there are some patients who have idiopathic pulmonary arterial hypertension and also have a bystander atrial septal defect. There have been several previous reports in which a fenestration has been created in a device to function as a “pop-off” valve in these circumstances.Reference Kenny, Cao and Hijazi 2 – Reference Dell'Avvocata, Rigatelli, Cardaioli and Giordan 7 These fenestrations often close in an unpredictable manner over a period of time. In an attempt to create a fenestration of predictable size, which may be resistant to early closure, we devised a technique of positioning a stent through an Amplatzer septal occluder (St. Jude Medical, St. Paul, Minnesota, United States of America). We present the case of a 6-year-old boy with an atrial septal defect and idiopathic pulmonary arterial hypertension in whom an Amplatzer septal occluder, fenestrated with a coronary stent, was implanted to reduce the degree of atrial shunting but maintain the fenestration in the long term.
Case report
A 6-year-old boy presented initially at the age of 2 years with idiopathic pulmonary arterial hypertension and a secundum atrial septal defect, for which he had been prescribed a combination of a calcium channel blocker (amlodipine), nocturnal oxygen, a phosphodiesterase-5 inhibitor (sildenafil) and an endothelin receptor antagonist (bosentan). His oxygen saturations were 98% on room air at rest and he had significant desaturation to 83% on 6-min walk test, managing a distance of 360 m.
Serial follow-up with echocardiography had revealed an estimated systolic pulmonary artery pressure of 70 mmHg and cardiac magnetic resonance imaging had estimated the ratio of right ventricle outflow to left ventricle outflow as 1.3:1. His atrial septal defect measured 12 mm and was shunting from left to right. Cardiac catheterisation at the age of 4 years had revealed a pulmonary vascular resistance of 12 Wood units × m2, reducing to 9 Wood units × m2 with pulmonary vasodilators.
It was decided after discussion with the national pulmonary hypertension service that, as there was no evidence of a significant fall in pulmonary vascular resistance with therapy, he would benefit from fenestrated closure of the atrial septal defect to reduce the left-to-right shunt and aid right ventricular remodelling. Owing to the fact that the idiopathic pulmonary arterial hypertension was unlikely to resolve completely following the atrial septal defect closure, the residual atrial communication would need to remain patent in the long term.
Under general anaesthesia, a transoesophageal echocardiogram was performed, which confirmed a 14 mm atrial septal defect with a deficient aortic rim. Sheaths were inserted into the left and right femoral veins and arterial access gained via the right femoral artery. A diagnostic catheter procedure was performed, which demonstrated a pulmonary to systemic flow ratio of 1.3:1 and a pulmonary vascular resistance index of 6.7 Wood units × m2. The pulmonary artery pressure was just sub-systemic with a mean of 43 mmHg compared with a mean aortic pressure of 47 mmHg.
The defect was sized in the standard manner with a 24 mm sizing balloon (St. Jude Medical). Fluoroscopy and transoesophageal echocardiography were used to determine the stop flow atrial septal defect diameter, which was found to be 16.3 mm. An 18 mm Amplatzer septal occluder device (St. Jude Medical) was modified and implanted in the following manner: a scalpel was used to cut a cross shape into the Gortex membrane in the central portion of the device (Fig 1a and b). A 0.014-inch 190 cm Balanced Heavy Weight coronary guide wire (Abbott, Santa Clara, California, United States of America) was fed through the hole and the device was loaded into the delivery system with the wire in place (Fig 1c–e). The delivery system used (10 F) was 2 French sizes larger than the recommended size for this device, to allow for any increased bulk and device deformation caused by having the additional coronary wire in place. The atrial septal defect was crossed in the standard manner and an Amplatzer superstiff wire positioned in the left upper pulmonary vein. The 10 F Amplatzer delivery sheath was then advanced into the pulmonary vein in the standard manner. After removing the dilator and superstiff wire, the device loader was connected onto the sheath (Fig 1f) and the coronary wire was advanced to sit in the left upper pulmonary vein (Fig 2a). The device was then advanced over the coronary wire with the wire fixed in the left upper pulmonary vein. The left atrial disc was deployed in the standard manner and the device was deployed across the defect maintaining the coronary wire position in the left upper pulmonary vein. Stable position was confirmed on transoesophageal echocardiography and fluoroscopy. With the device still attached to the delivery cable, a 16 mm-long by 5 mm-diameter Liberte monorail coronary stent (Boston Scientific, Natick, Massachusetts, United States of America) was introduced through the haemostatic valve over the coronary wire and manipulated into place to lie with 50% of its length in the left atrium and 50% in the right atrium through the device fenestration, using a steep left anterior oblique fluoroscopic angulation (Fig 3a) and transoesophageal echocardiography. The stent was then fully inflated in this position (Fig 3b). Transoesophageal echocardiography confirmed laminar flow through the stent (Fig 2b) which, under general anaesthesia, was from left atrium to right atrium. The coronary wire was then withdrawn through the stent (Fig 3).
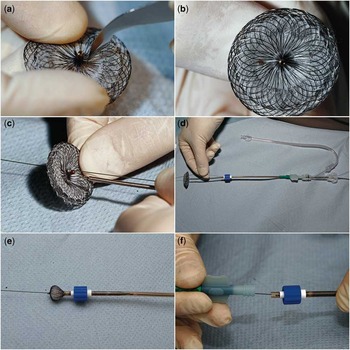
Figure 1 ( a and b ) Creating a hole in the central portion of the atrial septal occluder; ( c – e ) inserting coronary wire through hole and loading into delivery system; and ( f ) coronary wire being inserted into delivery sheath.

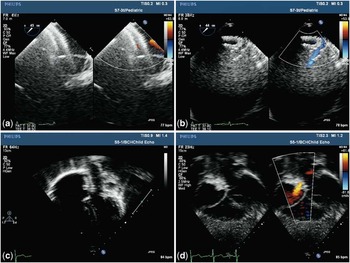
Figure 2 ( a ) Transoesophageal echocardiogram (TOE) with atrial septal occluder (ASO) deployed across atrial septal defect and coronary wire going through it; ( b ) TOE with ASO device in situ with stent in position – laminar flow through stent; ( c ) transthoracic echocardiogram (TTE) apical 4-chamber view with device and stent in situ; and ( d ) TTE subcostal 4-chamber view with device and stent in position and laminar flow through stent.

Figure 3 ( a ) Atrial septal occluder deployed with coronary stent being positioned across hole; ( b ) coronary stent being deployed with balloon; ( c ) atrial septal occluder device released with coronary wire still in position; and ( d ) device in position with stent maintaining hole.

Figure 4 Illustrative photographs of coronary stent deployed across atrial septal occluder device.
The patient was maintained on a heparin infusion until achieving a therapeutic international normalised ratio with warfarin (target of 2–3). At follow-up 1 month post-procedure, he had shown a marked improvement in his 6-min walk test, in which he managed to walk 240 m without significant desaturation (minimum saturation 93% in air). His parents felt that he had subjectively more energy and improved exercise tolerance. Echocardiogram demonstrated that the fenestration was still present with laminar left-to-right shunting. A further follow-up appointment at 7 months post-procedure again demonstrated the atrial communication to be patent, with the same flow pattern as seen previously. The warfarin was discontinued after 4 months, and he is currently taking aspirin to maintain the patency of the fenestration.
Discussion
Previous papers have outlined methods for creating fenestrated devices for atrial septal defect closure, but all have concentrated on that residual defect being present short term.Reference Kenny, Cao and Hijazi 2 – Reference Dell'Avvocata, Rigatelli, Cardaioli and Giordan 7 This is a useful strategy if the atrial pressures are liable to normalise medium-term after reduction of the left-to-right shunt, for example pulmonary hypertension secondary to increased flow or mitral regurgitation secondary to left atrial enlargement and eventual closure of the fenestration could be seen as desirable in these circumstances.
Our technique, which we believe to be the first published account of the creation of a long-term stented fenestration through an atrial septal defect occlusion device, is suitable for patients who are at risk of developing pulmonary hypertensive episodes. Allowing for a right-to-left shunt during these episodes may provide a “safety-valve”, allowing maintenance of left ventricular preload and cardiac output during episodes of increased pulmonary resistance. At the same time, significantly decreasing the magnitude of the left-to-right shunt by partial closure of the atrial communication may decrease one of the drivers of pulmonary hypertension in these cases.
It is difficult to quantify the increased thrombotic risk with this combined, modified device. To limit this risk, we chose the shortest stent that allowed a stable configuration through the deployed device, avoiding excessive “hang out” into either the left or the right atrium. We made an empirical decision to warfarinise the patient for at least the period of time required for endothelialisation of the devices and then to put him on aspirin.
Bench testing of the stent insertion before proceeding with the clinical application allowed us to select the optimal-sized stent, which was deemed to be 16 mm long for a 18 mm Amplatzer septal occluder device. This allowed us to be sure that a stable stent position could be obtained, with as little protrusion as possible into the left or right atrium. An uncovered stent was chosen to allow the maximum potential area for flow on either side of the device.
The technique for device modification is simple and reproducible and has produced the required anatomical and clinical result with successful maintenance of a long-term fenestration. It remains to be seen whether this process is beneficial for management of his idiopathic pulmonary arterial hypertension in the long term.