Sudden cardiac death in children is a devastating event. Paediatric sudden cardiac death occurs in an estimated 0.8–6.2 per 100,000 children annuallyReference Rodday, Triedman and Alexander1,Reference Mitani, Ohta and Yodoya2 . Therefore, heart screening in schools has been established in several countriesReference Rodday, Triedman and Alexander1,Reference Sumitomo, Baba and Doi3 . All children must undergo school heart screening at 6, 12, and 15 years of age in Japan. Diseases such as Wolff–Parkinson–White syndrome, hypertrophic cardiomyopathy, coronary artery anomalies, and long QT syndrome may lead to sudden cardiac death. Wolff–Parkinson–White syndrome is detected in 0.1–0.2% of patients during heart screening. The lifetime risk of sudden cardiac death in symptomatic Wolff–Parkinson–White syndrome is estimated at 3–4%Reference Arnar, Mairesse and Boriani4. Even in those with asymptomatic Wolff–Parkinson–White syndrome, electrophysiological studies for risk stratification may be considered in school children or athletes, as those with a shortest pre-excited RR interval of ≤ 250 ms during atrial fibrillation or rapid atrial pacing are at an increased risk of sudden cardiac death. Therefore, it is reasonable to consider catheter ablation in this groupReference Cohen, Triedman and Cannon5. However, once an invasive electrophysiological study is performed, moving forward to catheter ablation seems appropriate because electrophysiological study is an imperfect predictor and the risk association with ablation is lowReference Etheridge, Escudero and Blaufox6.
Wolff–Parkinson–White syndrome is usually diagnosed by the presence of delta waves on electrocardiography. In rare case, ventricular pre-excitation is not apparent during sinus rhythm despite existing anterograde accessory pathway conduction and has the possibility of rapid conduction of atrial fibrillation via the accessory pathway, resulting in ventricular fibrillationReference Robinson, Rowland and Krikler7 or sudden cardiac death. This latent form of Wolff–Parkinson–White syndrome is often diagnosed in symptomatic patients with palpitations, chest pain, or syncope. Subtle electrocardiography findings such as absent Q waves in the left precordial leads, presence of Q waves in V1, ST segment depressionReference Greenland, Kauffman and Weir8, or incomplete right bundle branch block may be noticed after making the diagnosis of a latent form of Wolff–Parkinson–White syndrome.
We report a 16-year-old girl with asymptomatic latent Wolff–Parkinson–White syndrome detected on school heart screening. This report demonstrates how it may be possible to detect latent Wolff–Parkinson–White syndrome in asymptomatic patients as part of the screening initiative.
Case presentation
A girl was referred to our hospital for an abnormal electrocardiogram found on school heart screening at 6 years of age. Small Q waves in lead V1, which suggested Wolff–Parkinson–White syndrome, cardiomyopathy, or coronary artery anomalies, were detected but due to normal echocardiographic and Holter electrocardiography findings, she was scheduled for repeat check-ups at 12 and 15 years of age. She did not have any symptoms or abnormal echocardiographic findings on these follow-ups. However, ST segment depression and small Q waves in lead V1 were detected during a high school medical check-up at the age of 16 years (Fig 1a). Resting electrocardiography showed no evident delta waves during sinus rhythm, but QRS morphology changed during premature atrial contractions, suggesting ventricular pre-excitation (Fig 1b). Treadmill testing at peak exercise and recovery exhibited ST-segment depression (Fig 1c). Echocardiography revealed normal findings. Adenosine triphosphate administration revealed manifest delta waves (Fig 1d). These findings suggested a latent form of left-sided atrioventricular accessory pathway. Although she was asymptomatic, electrophysiological study was performed under general anaesthesia to evaluate the risk of tachycardia and rapid ventricular response during atrial fibrillation, as it was difficult to interpret whether the ventricular pre-excitation was persistent. During sinus rhythm, the AH/HV intervals were normal (76/36 ms) (Fig 2a). During right atrial burst pacing, the ventricular pre-excitation was overt and the His electrogram was buried in the ventricular electrogram (Fig 2b). The shortest pre-excited RR interval during rapid atrial pacing was 375 ms at baseline and the effective refractory period of the accessory pathway was 360 ms at baseline and 270 ms during isoproterenol infusion. Ventriculoatrial conduction was only via the atrioventricular node in both settings. Premature atrial contraction due to catheter manipulation induced antidromic atrioventricular reciprocal tachycardia at a rate of 180 bpm (Fig 2c). Given the relatively short shortest pre-excited RR interval during rapid atrial pacing on isoproterenol infusion and inducible antidromic atrioventricular reciprocating tachycardia (class IIb indication by PACES/HRS expert consensus statement), ablation was performed. Furthermore, accessory pathway ablation was considered safe because the ablation site was far from the atrioventricular node. Therefore, the accessory pathway was ablated on the left lateral mitral annulus (Supplemental Figure). After ablation of the accessory pathway, the 12-lead electrocardiogram showed no delta waves during coronary sinus pacing. The accessory pathway conduction did not recur after 8 years.

Figure 1. ( a ) Surface ECG during school heart screening showing: ST segment depression in lead aVF and small Q waves in lead V1. ( b ) Twelve-lead ECG: No delta waves are observed in the first 2 beats. Small Q waves are noted in lead V6. No ST-segment depression is seen. However, the QRS morphology changes after the PAC. The PR interval is short and the QRS interval wide, suggesting a delta wave. ( c ) Treadmill exercise test: ST segment depression noted in all leads except aVR during exercise and recovery. ( d ) Adenosine triphosphate administration: apparent delta waves (*) follow P-waves.

Figure 2. ( a ) Intracardiac electrogram (ICE) during sinus rhythm: AH/HV interval: 76/36 ms, the earliest ventricular activation site is coronary sinus (CS) 3–4, and the distance between the atrial electrogram and ventricular electrogram is very long. ( b ) On ICE imaging during atrial burst pacing at a rate of 160 ppm, the His electrograms are buried in the ventricular electrogram. The ventricular electrograms in the His and RVA recordings are delayed. The surface ECG exhibits manifest delta waves. ( c ) Antidromic atrioventricular tachycardia: The heart rate is 180 bpm and a right bundle branch block pattern in lead V1 and negative waves in lead I are noted on the surface ECG, and the earliest ventricular site is recorded at the left lateral mitral annulus by the ABL catheter. The earliest atrial site is the His bundle.
Discussion
A patient with asymptomatic latent Wolff–Parkinson–White syndrome was found during school heart screening due to the presence of Q waves in lead V1 and ST segment depression. Ventricular pre-excitation was not overt during sinus rhythm but became apparent during atrial pacing, premature atrial contractions, or adenosine triphosphate administration. The shortest pre-excited RR interval during rapid atrial pacing with an isoproterenol infusion was relatively short, and antidromic atrioventricular reciprocal tachycardia was induced. The left lateral accessory pathway was successfully ablated, and no Q waves in lead V1 or ST segment depression were observed thereafter. This latent form of Wolff–Parkinson–White syndrome has usually been diagnosed in symptomatic patients with palpitations, chest pain, or syncope. Although it is difficult to diagnose in asymptomatic patients, it may be possible to detect latent Wolff–Parkinson–White syndrome in asymptomatic patients as part of a screening initiative.
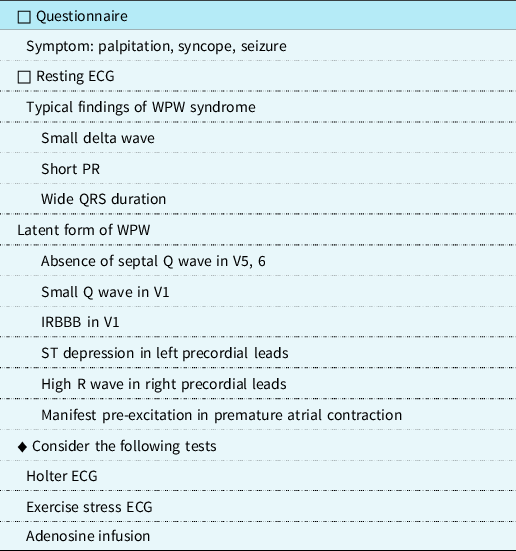
Concern about sudden cardiac death has raised calls for screening in primary care or school-based settings for all children. Japan and Taiwan perform mass screening of school-age children, including a targeted cardiac history, a physical examination, and electrocardiogram. However, cardiac screening for those subgroups of children being started on stimulants or participating in competitive athletics has been performed in many countriesReference Rodday, Triedman and Alexander1. “The Guidelines for School Heart Screening in SchoolsReference Sumitomo, Baba and Doi3” and the “International Recommendations for Electrocardiographic Interpretation in AthletesReference Sharma, Drezner and Baggish9” have been published. Wolff–Parkinson–White pattern was defined as a PR interval <120 ms, presence of delta waves, and QRS duration >120 ms. Pathologic Q waves in lead V1 (a Q/R ratio ≥0.25 or ≥40 ms in duration) or a qR pattern in lead V1 and ST-segment depression in excess of 0.05 mV in two or more leads are abnormal findings and need further investigationReference Goldberger10 (Table 1). In latent Wolff–Parkinson–White syndrome, QRS and ST segment changes can be subtle. In our case, a PR interval of 160 ms, very small Q waves in lead V1, and a slight ST-segment depression (≤0.05 mV) were observed during screening. Her electrocardiogram recorded in our hospital revealed no QV1 or ST segment depression during sinus rhythm but an incidental premature atrial contraction induced manifest delta waves suspicious of Wolff–Parkinson–White syndrome. Holter electrocardiograms and exercise testing helped diagnose latent Wolff–Parkinson–White syndrome. Furthermore, the accessory pathway was diagnosed by the administration of adenosine triphosphate before the electrophysiological study.
Table 1. Clinical evaluation to detect latent WPW syndrome.
If the time taken for the propagation of a sinus impulse to and across the accessory pathway is greater than the time taken for that impulse to propagate to the ventricles via the atrioventricular node, pre-excitation may not be observed. Such conditions may occur with a left lateral pathway, delay in the intra-atrial conduction time, increased left atrial size, or accelerated atrioventricular nodal conductionReference Robinson, Rowland and Krikler7. This patient had a left lateral accessory pathway with a long conduction time and subtle decremental property.
In conclusion, we were able to diagnose asymptomatic latent Wolff–Parkinson–White syndrome during school heart screening. Careful diagnosis and ablation may have the potential to prevent malignant arrhythmias.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122000233
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency or commercial or not-for-profit sector.
Conflict of interest
None.






