Dextro-transposition of the great arteries with multiple ventricular septal defects presents a challenging surgical scenario; muscular defects can be hidden within tight right ventricular trabeculations making them surgically inaccessible. Traditionally, this necessitates palliative pulmonary artery banding post-arterial switch or an extensive ventriculotomy. Both are associated with significant long-term morbidity. During planning of this procedure, discussion between the interventional cardiologists and cardiac surgeons allowed coordination of a hybrid approach, combining simultaneous surgical and catheter based interventions.Reference Kang 1 These are becoming increasingly complex, providing results that are often greater than the sum of their parts.
Case Report
A female neonate with an antenatal diagnosis of dextro-transposition of the great arteries and perimembranous and apical muscular ventricular septal defects underwent an atrial septostomy at birth. As the muscular defect was large, the left ventricle remained at systemic pressures and there was therefore no risk of it regressing. Her haemodynamics remained stable, she continued to grow and her operation was planned at 5 months of age to maximise the likelihood of success.
At the joint cardiac conference, it was decided that, following her arterial switch operation, attempts should be made to close all of her ventricular septal defects surgically. These would then be assessed off bypass by trans-oesophageal echocardiography. Should there be an on-going significant shunt not amenable to surgical closure, she would then undergo an off-bypass per-ventricular device muscular ventricular septal defect closure.
An arterial switch without Lecompte manoeuvre was performed and the coronary arteries re-implanted into the neo-aortic root using a trap door technique. The perimembranous defect was closed and attempts were made at muscular defect closure via the pulmonary valve with a pledgeted prolene suture. The right ventricular outflow tract was then reconstructed with a pericardial patch, the atrial septal defect closed, and the patient weaned from bypass. The trans-oesophageal echocardiogram demonstrated a residual 4 mm muscular defect with significant left to right shunting (Fig 1a). As per the pre-operative plan, the decision was collectively taken to close this using a per-ventricular device.
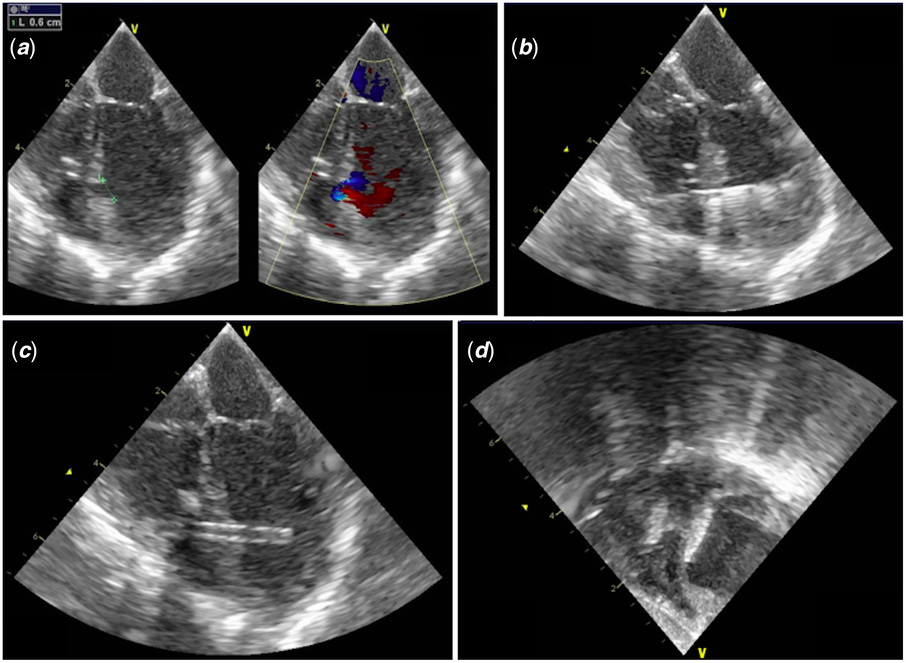
Figure 1. Trans-oesophageal images: (a) Mid-oesophageal four-chamber view showing a mid-trabecular muscular VSD measuring 6 mm. (b) Mid-oesophageal four-chamber view with a 0.035 J-Tip wire through the VSD to the left ventricle. (c) Mid-oesophageal four-chamber view showing the 6Fr short sheath through the VSD to the left ventricle. (d) Trans-gastric view showing a 6 mm VSD Occluder device in a good position with well-deployed left and right ventricular discs.
After choosing the access site with finger pressure under echo guidance, a purse string was positioned to the right ventricular body, which was then punctured and the defect cannulated with a 0.035 J-tip wire (Fig 1b). A 6-French short sheath was used to deploy a 6 mm Amplatzer muscular ventricular septal defect device (Fig 1c). Trans-oesophageal echocardiography prior to release demonstrated correct placement with only a mild residual shunt just above the left ventricular disc (Fig 1d). The sheath was removed and the purse string tightened. The bypass, cross clamp, and catheterisation times were 206, 180, and 10 minutes, respectively.
The patient required 48 hours of inotropic and ventilatory support and peritoneal dialysis before being extubated and transferred to the cardiac ward the following day. She was discharged home 5 days later. At 6 weeks, she was well and thriving; her echocardiogram showed an excellent repair with a well-placed device and a tiny, clinically insignificant residual left to right shunt.
Discussion
The presence of muscular ventricular septal defects in complex cardiac anatomy, such as the lesion presented here, is an independent risk factor for early mortality.Reference Bacha and Hijazi 2 Common practice is to perform an arterial switch and then attempt to close the defects surgically. The position of muscular defects often presents a challenge in small patients; the only surgical options are a right ventriculotomy or pulmonary artery band placement, which protects the pulmonary vasculature from over-circulation while the child grows and allows easier surgical access for a future closure. The latter necessitates at least one more bypass operation and on-going diuretics before this. Data from adolescent and adult patients have demonstrated chronically reduced right ventricular function and exercise capacity following band removal.Reference Braun, Szalai, Strasser and Borst 3
Ye et al published a cases series that included one arterial switch and per-ventricular muscular ventricular septal defect closure. It failed due to impingement of the anterior mitral valve leaflet and the device was removed.Reference Ye, Jiang, Zhang, Li and Shu 4 Kim et al published a similar case in a 25-day-old patient, which was complicated by sheath displacement before device deployment and a secondary tear to the left ventricular wall requiring surgical closure.Reference Kim 5
More recently, Michel-Behnke et al published a series of four arterial switches with echocardiographically guided per-ventricular muscular ventricular septal defect device closures in neonates aged 0.2–0.3 months.Reference Michel-Behnke 6 At a mean follow-up of 37.1 weeks, all patients were alive: one had an on-going moderate left to right shunt; two had none; and one developed complete heart block, requiring removal of the device.
The technique as we have described has several advantages beyond alleviating all of the aforementioned risks of an arterial switch procedure and pulmonary artery banding. When compared to fluoroscopically guided transcatheter device placement, echocardiographic guidance limits radiation and nephrotoxic contrast exposure and the need for transfer to a catheterisation lab. Other reports have used a straight wire passed through the sheath to cannulate the defect, which can be difficult to visualise on ultrasound, risking injury to the septum or conducting system. The J-tip wire used here mitigated these risks. One alternative approach is to do a pre-operative trans-septal ventricular septal defect device closure via the femoral vein. However, it was thought that this would have been higher risk in this case.
The pre-operative multi-disciplinary coordination required for this procedure cannot be over-stated. However, this coordination reduces the total number of operations required in patients where single-stage full surgical repair is not feasible. This provides important benefits to both patients and health care systems at a time when large numbers of operations in the United Kingdom have been cancelled due to bed pressures.
Conclusion
This case and the supporting literature demonstrate the feasibility, safety, and advantages of this hybrid procedure as an alternative to arterial switch and pulmonary artery banding in infants with this lesion. The authors suggest that it should be considered in appropriate patients where hybrid facilities are available. As demonstrated by the complications from previous reports, pre-operative coordination, and appropriate patient, device size and ventricular puncture site selection are crucial to its success.
Acknowledgements
None.
Financial Support
This report received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
Not applicable.



