Apical hypertrophic cardiomyopathy is an uncommon morphologic variant of hypertrophic cardiomyopathy that predominantly affects the apex of the left ventricle, characterised by giant negative T waves on the electrocardiogram and spade-shaped left ventricle cavity.Reference Jan, Todaro, Oreto and Tajik 1 Since it is typically diagnosed in midlife, the vast majority of current reports of apical hypertrophic cardiomyopathy are confined to adults,Reference Zhai, Fan and An 2 – Reference Moro, D’Angelo, Nicolosi, Mimo and Zanuttini 4 with very few cases in children,Reference Miyamoto, Horigome, Kawano and Sumazaki 5 all of whom were asymptomatic and detected incidentally due to abnormal electrocardiogram findings at a routine health check-up. We present a school-age boy who presented with exertional chest pain found to have apical hypertrophic cardiomyopathy.
Case report
A 9-year-old boy was admitted to our hospital secondary to a 2-week history of chest pain. The chest pain localised to the left chest which was precipitated by exertion, but recently occurred more frequently and occurred with both with activity and rest. The patient denied any chest trauma, he had not experienced syncope and had no family history of cardiac disease. Physical examination was within normal limits. Laboratory data revealed a normal complete blood count, biochemical parameters, creatine kinase, brain natriuretic peptide, and cardiac troponin I. Chest radiograph was negative. Electrocardiogram revealed constant giant negative T waves with ST depression in the anterior and lateral leads (Fig 1), which was unrelated to the presence of chest pain and showed no resolution in the next few days. However, the initial echocardiography did not suggest cardiomyopathy or coronary artery abnormalities.

Figure 1. Electrocardiogram shows a loss of septal Q waves, high QRS voltage, and repolarisation abnormalities with deep T wave inversion, especially in leads V3 and V4 (black arrow). Such abnormalities are also evident in inferior and lateral leads.
Cardiac angiography was performed to further evaluate cardiac causes of chest pain and abnormal electrocardiogram. Coronary angiography demonstrated enlarged epicardial left coronary artery without focal stenosis and myocardial bridging. Left ventricle angiography was suggestive of a spade-like appearance, which is characteristic of apical hypertrophic cardiomyopathy (Fig 2). MRI confirmed the findings, and large areas of delayed enhancement were consistent with apical fibrosis (Fig 2). After focusing on the myocardium of left ventricle apex, a second echocardiography revealed a progressive increase in left ventricle wall thickness towards the apex without evidence of outflow tract or midventricular obstruction. Left ventricle end-diastolic dimension at the level of the mitral valve leaflet tips was 40 mm with an ejection fraction of 68%. The end-diastolic thickness of left ventricle apical and basal free wall was 17 and 8.5 mm, respectively. The end-diastolic thickness of apical and basal interventricular septum was 12 and 8.2 mm, respectively. Continuous electrocardiogram monitoring during hospitalisation found no significant arrhythmias and a 24-hour Holter monitor only showed occasional uniform premature ventricular contractions. His parents also underwent echocardiography and electrocardiogram, both of which were normal.
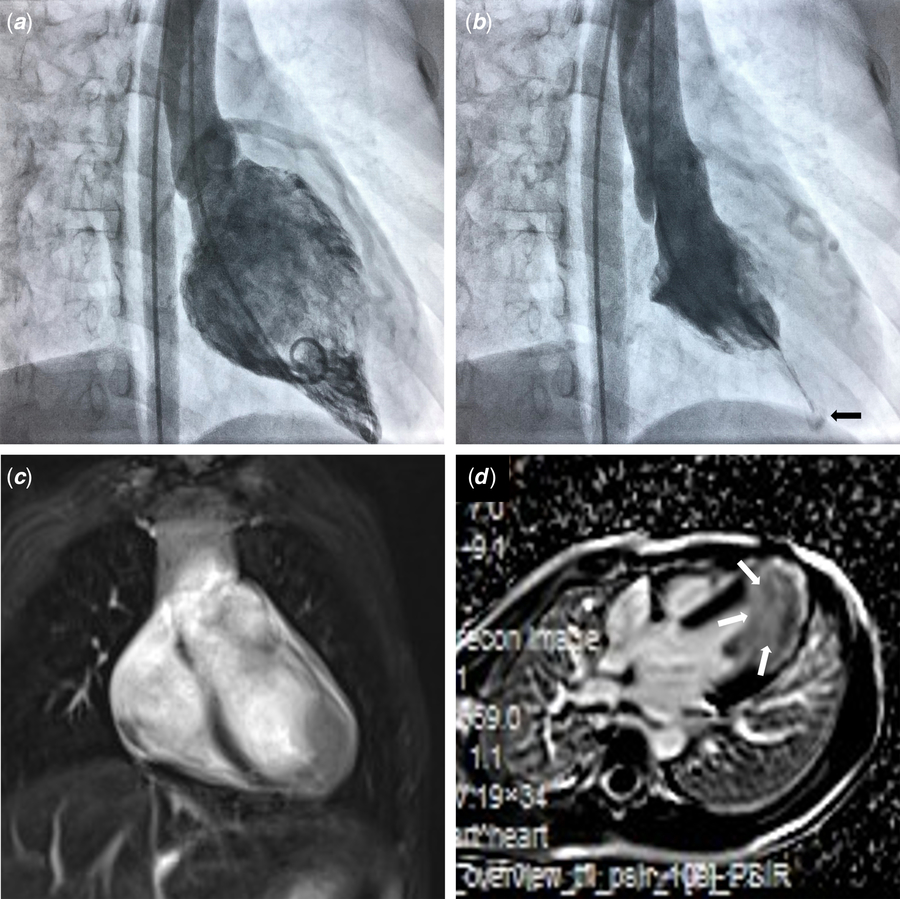
Figure 2. Left ventricle angiography in the right anterior oblique view and cardiac MRI. ( a ) The typical spade-like appearance at end-diastole. (b ) During end-systole, a small cavity in the apical portion (black arrow) was separated from the basal cavity by long cavity obliteration. (c,d) Cardiac MRI with gadolinium enhancement demonstrating focal apical hypertrophy and fibrosis (white arrows).
Though he had not yet undergone confirmatory genetic testing, the presence of apex-predominant left ventricle hypertrophy with an ace-of-spades configuration of the left ventricle cavity, and giant negative T waves, strongly suggested apical hypertrophic cardiomyopathy as the aetiology of his chest pain. On admission day seven, our patient was discharged home on metoprolol, and he remained symptom-free at 1-month follow-up.
Discussion
Since apical hypertrophic cardiomyopathy was first reported in Japan 40 years ago, a growing number of literatures have described its clinical features,Reference Jan, Todaro, Oreto and Tajik 1 helping clinicians recognise that apical hypertrophic cardiomyopathy may be a commonly missed or delayed diagnosis, especially when patients present with acute chest pain and an abnormal electrocardiogram.
Given the rarity of apical hypertrophic cardiomyopathy diagnosed in childhood and the fact that apical imaging is not an advantage of echocardiography, a paediatric cardiologist would first consider anomalous coronary artery origin or coronary ischemia when the patients presented with chest pain and an abnormal electrocardiogram but no indication of cardiomyopathy at the initial echocardiography.Reference Friedman and Alexander 6 After a careful review of literature, we found only four cases of apical hypertrophic cardiomyopathy in children younger than 18 years who were asymptomatic,Reference Miyamoto, Horigome, Kawano and Sumazaki5, Reference Chaowu, Shihua, Jian, Li and Wei7 and no paediatric cases were reported in other apical hypertrophic cardiomyopathy studies.Reference Zhai, Fan and An 2 – Reference Moro, D’Angelo, Nicolosi, Mimo and Zanuttini 4 Therefore, to the best of our knowledge, this is the first reported case of apical hypertrophic cardiomyopathy presenting with chest pain in children.
The trademark electrocardiogram finding in apical hypertrophic cardiomyopathy is giant negative T waves (≥10 mm) in the left precordial leads associated with high QRS voltage, which were found more commonly in Japanese patients (64%) as compared with the United States of America (30%).Reference Jan, Todaro, Oreto and Tajik 1 Myocardial ischemia can also be manifested as ST-T abnormalities, but ST-segment depression and T wave inversion are generally concordant to low-voltage QRS complex.Reference Hanna and Glancy 8 Though Sakamoto summarised 10 years of clinical data and found no giant negative T in children younger than 15 years,Reference Sakamoto, Amano and Hada 9 we now know that this is not the case, and that characteristic electrocardiogram findings are sometimes more sensitive than echocardiography for diagnosing apical hypertrophic cardiomyopathy. MRI may facilitate a more comprehensive evaluation of hypertrophic segments and myocardial fibrosis.
Similar to classic hypertrophic cardiomyopathy, current studies have demonstrated a familial association with apical hypertrophic cardiomyopathy including an autosomal dominant inheritance pattern. Several hundred mutations have been identified, scattered among at least 27 hypertrophic cardiomyopathy susceptibility genes encoding various sarcomeric and non-sarcomeric proteins. Generally, the apical hypertrophic cardiomyopathy was most commonly associated with mutations in MYBPC3, MYH7, ACTC1, and TPM1.Reference Jan, Todaro, Oreto and Tajik 1
We hope that this case will enhance paediatrician’s awareness of the characteristic electrocardiogram and imaging findings of apical hypertrophic cardiomyopathy and help in the early identification of this rare cause of paediatric chest pain.
Acknowledgements
The authors thank their colleagues in radiology department for the exquisite cardiac MRI images.
Financial Support
Key Developing Discipline of Shanghai Municipal Commission of Health and Family Planning (Pediatrics) 2016ZB0101.
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and were approved by the Ethics Committee of the Children’s Hospital of Fudan University.




