Published online by Cambridge University Press: 14 April 2005
We report a girl, aged 11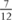 years, who presented with cyanosis. Cardiac catheterization showed occlusion of the infrahepatic segment of the inferior caval vein, with drainage of the hepatic veins into the left atrium. Transoesophageal echocardiography revealed an anomalous Eustachian valve that baffled the vein to the left atrium. This lesion is an extremely rare cause of cyanosis.
years, who presented with cyanosis. Cardiac catheterization showed occlusion of the infrahepatic segment of the inferior caval vein, with drainage of the hepatic veins into the left atrium. Transoesophageal echocardiography revealed an anomalous Eustachian valve that baffled the vein to the left atrium. This lesion is an extremely rare cause of cyanosis.
Connection of the inferior caval vein to the morphologically left atrium is extremely rare. When seen, most cases are associated with atrial septal defects.1, 2 In these patients, it is the occurrence of severe cyanosis despite the presence of the atrial septal defect with normal right ventricular pressures that points to the correct diagnosis. Indeed, drainage of the inferior caval vein to the left atrium should always be considered as a potential diagnosis when confronted with the triad of cyanosis, clubbing of the fingers and toes, and the absence of other pathological findings in the cardiac examination.3, 4 In this report, we describe an even rarer case in which the atrial septum seemed to be intact.
An 11

-year-old girl was admitted to our unit with a history of exercise-induced cyanosis of her lips. A marked cutaneous network of venous vessels on the body and arms had been noted since the first year of life. She was born at 33 weeks of gestation, and underwent surgery postnatally for correction of jejunal atresia.
Physical examination revealed mild cyanosis of her lips, clubbing of the fingers and toes, and cutaneous venous distension on the body and arms. There were no cardiac murmurs, nor signs of left or right ventricular failure. The haematocrit was measured at 44 percent, while the saturation of oxygen established transcutaneously was 87 percent. A chest X-ray showed a normal cardiac silhouette and pulmonary vasculature. The electrocardiogram was unrevealing.
At first impressions, transthoracic echocardiography showed normal cardiac morphology and function. Studies administering contrast from the arms demonstrated normal opacification of the right atrium and ventricle. Contrast given from the left lower leg, however, showed delayed and weak opacification of the right atrium via the superior caval vein, with no contrast entering the right atrium through the inferior caval vein.
Cardiac catheterization performed via the femoral vessels revealed occlusion of the infrahepatic caval vein, drainage from the body via paravertebral collateral vessels and the azygos vein into the superior caval vein, and drainage of the hepatic veins via the suprahepatic segment of the inferior caval vein into the left atrium (Fig. 1). The pressures in the right heart were normal. The pulmonary venous saturation, of 94.8 percent, decreased to 91.3 percent in the left atrium, and was not elevated by inhalation of 100 percent oxygen.

Figure 1. Angiogram of the left atrium in anteroposterior (a) and lateral views (b). The catheter passes from the aorta into the left ventricle and, via mitral valve, into the left atrium. White arrowheads denote contrast passing through the inferior caval vein towards the hepatic veins.
Transoesophageal echocardiography revealed an anomalous Eustachian valve fused with the upper part of the atrial septum, resulting in the inferior caval vein draining exclusively into the left atrium.
An operation was performed by midline sternotomy, incising the lateral wall of the right atrium. No communication was found between the right atrium and the inferior caval vein. Opening the region of the oval fossa showed that a large persistent Eustachian valve formed the inferior part of the tissues separating the atriums, and this was resected. The orifice of the inferior caval vein was localised on the left side of this interatrial wall, but was bordered on the left by a fold of the true interatrial septum, which was also resected. An oval pericardial patch inserted between the inferior caval vein and left atrium served to redirect the inferior caval venous blood into the right atrium (Fig. 2). Histopathological investigation of the resected interatrial membrane failed to show any features of postnatal fusion of the Eustachian valve with the atrial septum. The postoperative period was uneventful, marked by normalisation of the blood gases and regression of symptoms.

Figure 2. (a) The scheme shows the usual arrangement of the atrial septum: right (RA) and left atriums (LA) are divided by the interatrial septum, which exhibits a patent oval foramen (PFO). The orifice of the inferior caval vein (ICV) is bordered by the Eustachian valve (EV). The superior caval vein (SCV) drains directly into the right atrium. (b) The preoperative arrangement in our patient. A large Eustachian valve formed a tunnel that directed the blood from the inferior caval vein through an atrial septal defect (ASD) into the left atrium. The orifice of the inferior caval vein was bordered on the left by a fold of interatrial septum. (c) The postoperative situation. A pericardial patch (PP) is placed to redirect the inferior caval venous blood into the right atrium.
The differential diagnosis in patients presenting with cyanosis as the only symptom includes congenital or acquired pulmonary arteriovenous fistula, pulmonary arterial connection to the left atrium, and anomalous systemic venous connection to the left atrium, as for example, when an inferior sinus venosus atrial defect is associated with inferior caval venous flow into the left atrium. Contrast echocardiography is useful in distinguishing between these differential diagnoses.5 In our patient, such injection of contrast material excluded pulmonary arteriovenous malformations, as well as an anomalous connection of the superior caval vein. It also suggested venous drainage through the azygos system, since contrast injected into the foot produced a delayed but weak opacification of the right atrium via the superior caval vein. This was, however, potentially misleading, since subsequent cardiac catheterization showed that the infrahepatic segment of the inferior caval vein was thrombosed, and it was only the drainage of the lower part of the body that passed through paravertebral collateral channels, the azygos venous system and the superior caval vein to reach the right atrium. Our catheterization also revealed that the suprahepatic portion of the inferior caval vein was connected anomalously to the left atrium.
This finding, of course, meant that hepatic blood was no longer able to traverse the lungs. This is important, since the risk of pulmonary arteriovenous malformations is now well recognized in this setting.3 Furthermore, it is known that these acquired pulmonary arteriovenous malformations resolve after surgical redirection of hepatic venous flow to the right heart and lungs.3, 6 Surgical repair of isolated drainage of the inferior caval vein into the left atrium is indicated, therefore, not only for prevention of paradoxical embolism, improvement of exercise intolerance, resolution of low arterial oxygen saturation and polycythemia, but also to prevent the development of pulmonary arteriovenous malformations.
In isolation, congenital connection of the inferior caval vein into the left atrium is an extremely rare cardiac malformation. Doven et al.7 reported a case with a left-sided inferior caval vein associated with sinus venosus atrial defect and anomalous drainage of pulmonary veins. Brochard et al.8 presented a case of infrahepatic interruption of the caval vein with azygos continuation and left-sided hepatic venous drainage. In the absence of abnormalities of lateralization, however, it is difficult to offer a sensible embryological explanation to connection of the right-sided inferior caval vein into the morphologically left atrium. Thus, most case reports of such anomalous connection of the inferior caval vein to the left atrium have described an abnormally large right valve of the systemic venosus sinus (“sinus venosus”) coexisting with an atrial septal defect or patent oval foramen.1, 2, 4 During early embryologic development, the right and left venous valves separate the systemic venous sinus from the primary part of the right atrium.9 The left venous valve subsequently fuses with the atrial septum. As the systemic venous sinus becomes incorporated into the right atrium, so the right venous valve is resorbed. Its caudal remnant becomes divided into the Eustachian and Thebesian valves. With this in mind, it is easy to hypothesize that, in our patient, a large Eustachian valve persisted to form a tunnel, which channeled all the inferior caval venous blood into left atrium. Our patient, however, was born with another malformation, namely jejunal atresia. Both intracardiac and intestinal anomalies may have originated in the same gestational time window. The histopathological investigation of the resected interatrial membrane supports the diagnosis of a congenital cardiac malformation.
Anomalous drainage of the inferior caval vein, nonetheless, is also recognized as an acquired event, as for example after inappropriate surgical repair of atrial septal defects.10 In these cases, the Eustachian valve may be mistaken for the lower margin of the defect. It is also possible to produce a hypothesis for an acquired genesis of the malformation in our case. In the fetal circulation, the Eustachian valve directs blood from the inferior caval vein through the patent oval foramen and into the left atrium. When our patient postnatally underwent corrective surgery of the jejunal atresia, intravenous medication was administered through an umbilical venous catheter. The presence of this catheter in the inferior caval vein may have set the scene for both thrombotic occlusion of the infrahepatic caval vein, and postnatal fusion of the Eustachian valve with the interatrial wall, thus directing the hepatic venous blood into the left atrium.
Anomalous drainage of the inferior caval vein into the left atrium, therefore, is a very rare cardiac malformation. It is almost always due to abnormal persistence of the right valve of the sinus venosus, baffling the inferior caval venous blood across the atrial septum and into the left atrium. The malformation should always be considered as a potential diagnosis in patients with cyanosis in the absence of elevated right atrial pressures.

Angiogram of the left atrium in anteroposterior (a) and lateral views (b). The catheter passes from the aorta into the left ventricle and, via mitral valve, into the left atrium. White arrowheads denote contrast passing through the inferior caval vein towards the hepatic veins.

(a) The scheme shows the usual arrangement of the atrial septum: right (RA) and left atriums (LA) are divided by the interatrial septum, which exhibits a patent oval foramen (PFO). The orifice of the inferior caval vein (ICV) is bordered by the Eustachian valve (EV). The superior caval vein (SCV) drains directly into the right atrium. (b) The preoperative arrangement in our patient. A large Eustachian valve formed a tunnel that directed the blood from the inferior caval vein through an atrial septal defect (ASD) into the left atrium. The orifice of the inferior caval vein was bordered on the left by a fold of interatrial septum. (c) The postoperative situation. A pericardial patch (PP) is placed to redirect the inferior caval venous blood into the right atrium.