Even though, as stated by Lev and Eckner,Reference Lev and Eckner1 hearts from no two patients are exactly the same, it is the characteristic anatomy that permits the instant recognition of the tetralogy of Fallot. The characteristic morphology had been recognised and illustrated long before Fallot realised that a constellation of 4 anatomic lesions was seen in the majority of specimens coming into his autopsy service from patients with “la maladie bleue”.Reference Fallot2 According to Marquis,Reference Marquis3 it was the Danish monk, Steno, also known as Stensen, who first described the association of lesions, having observed the findings in an ectopic heart from a fetus. More recently, attempts have been made to identify one of the four anatomic features as being paramount. Thus, someReference Van Praagh, Van Praagh, Nebesar, Muster, Sinha and Paul4 have argued that it is underdevelopment of the subpulmonary infundibulum that was the primary feature, stating that this part of the heart was “too narrow, too shallow, and too short”. Whilst the infundibulum is unequivocally narrow and hypoplastic, measurements of its length in series of hearts,Reference Becker, Connor and Anderson5, Reference Howell, Ho, Anderson and Elliott6 when compared to the length of the normal infundibulum, have shown that, rather than being too short, the infundibulum is often longer than normal. It can, nonetheless, be short, or even absent, in selected specimens, as we will show in our subsequent discussions. In our own early studies, we suggested that anterocephalad deviation of the insertion of the muscular outlet septum relative to the septomarginal trabeculation was the key feature.Reference Becker, Connor and Anderson5 This hypothesis was confounded by two observations. First, that the outlet septum could be deviated in antero-cephalad fashion without producing subpulmonary obstruction, as in the Eisenmenger defectReference Eisenmenger7. Second, that in some hearts, there can be subpulmonary obstruction when the outlet septum is fibrous, rather than muscular. Our further studiesReference Anderson and Weinberg8 have now identified anatomic features that do prove pathognomonic for the lesion. These involve anterocephalad deviation of the outlet septum, be it muscular or fibrous, but require an associated malformation of the septoparietal trabeculations, these being the muscular bars which reinforce the parietal wall of the right ventricle. It is on the basis of the squeeze produced between the malaligned outlet septum, or its fibrous remnant, and the septoparietal trabeculations that we now identify the morphologic entity of tetralogy of Fallot. In the discussions that follow, we will show how the building blocks of the normal right ventricular outflow tract become divorced one from the other in the setting of tetralogy of Fallot. Recognition of these building blocks as individual entities permits distinction of the clinically significant variations to be found in the lesion.
The building blocks of the normal subpulmonary infundibulum
In the normal heart, there is an extensive muscular area in the roof of the right ventricle separating the attachments of the leaflets of the tricuspid valve in the ventricular inlet, and those of the pulmonary valve in the ventricular outlet. This area is usually described as the supraventricular crest, or crista supraventricularis. The greater part of this structure is made up of the musculature of the inner heart curvature. Incisions through this part of the wall take the dissector outside the heart, revealing the location of the aortic root, and the right coronary artery. Careful dissection shows that a small part of the musculature, inserting between the limbs of the prominent muscular strap that reinforces the right ventricular septal surface, can be removed so as to create a passage into the left ventricle (Fig. 1). The area producing the passage to the left ventricle represents the muscular component of the septum. In malformed hearts, we describe this structure as the outlet septum. In the normal heart, however, this small area is so well incorporated into the overall musculature that, without dissection as shown in Figure 1, it is not possible to distinguish anatomically discrete areas within the union of the supraventricular crest and the septal components. Since the dissection takes the prosector into the left ventricle, it would be inappropriate to describe the removed area as being supraventricular, the communication with the left ventricle proving its interventricular position. It also follows that the muscular strap reinforcing the septal surface is interventricular rather than supraventricular (Fig. 2).
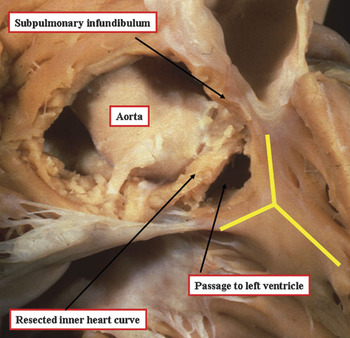
Figure 1 The area of the roof of the normal right ventricle interposed between the attachments of the leaflets of the tricuspid and pulmonary valves has been removed, showing that its greater part is the inner heart curvature. Removal of this area reveals the aortic root and the right coronary artery. The distal part supports the free-standing subpulmonary infundibulum. Note, however, that a small part of the septum has been resected so as to produce a passage into the outflow tract of the left ventricle. This part of the musculature is inserted between the limbs of the prominent muscular strap reinforcing the septal surface of the ventricle, shown in the picture by the yellow “Y”.
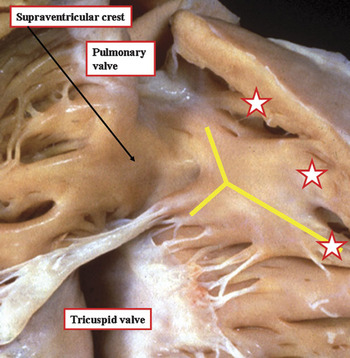
Figure 2 The subpulmonary outflow tract of the normal heart is opened to show the supraventricular crest, defined as the muscular area separating the attachments of the tricuspid and pulmonary valves in the roof of the right ventricle. The crest inserts between the limbs of the extensive muscular strap reinforcing the right ventricular septal surface, shown by the yellow “Y”. Note also the muscular bars that run from the cephalad margin of the strap to the parietal wall of the ventricle, marked by the stars.
In North America, it is customary to describe this part as the septal band of the crista. Since the crest should strictly be a supraventricular structure, septal components, or the muscular straps that reinforce them, should not logically be described as being septal. In the past, significant problems were created when the alleged components of the crest were used in the description of congenitally malformed hearts. Because of all these problems, it was suggested some time ago that the term supraventricular crest be reserved for description of the roof of the right ventricle in the normal heart.Reference Anderson, Becker and Van Mierop9 This was because the component parts of the right ventricular outflow tract could be identified in their own right only when this part of the heart was congenitally malformed. The inner heart curve itself was nominated as the ventriculo-infundibular fold, representing any muscular structure interposing between the attachments of the leaflets of the atruioventricular and arterial valves. Any muscular or fibrous structure interposing between the leaflets of the arterial valves themselves would obviously separate also the subarterial outlets, so this entity was defined as the outlet septum. The muscular strap reinforcing the septal surface, which has antero-cephalad and postero-caudal limbs, along with a body that extended into the apical trabecular component, was named the septomarginal trabeculation. The multiple muscular bundles that extended from the cephalad margin of the septomarginal trabeculation, and ran onto the parietal wall of the outflow tract, following the precedent set by Goor and Lillehei,Reference Goor and Lillehei10 were designated as the septoparietal trabeculations. The distal part of the subpulmonary infundibulum also exists in its own right. It, too, requires separate description. This part is the muscular sleeve that is removed, along with the leaflets of the pulmonary valve, to create the autograft used in the Ross procedure (Fig. 3). A representation of these building blocks of the normal right ventricular outflow tract is shown in Figure 4.

Figure 3 The pulmonary valve and its supporting infundibular sleeve has been removed from this normal heart, showing the space between the back wall of the infundibular sleeve and the aortic root (yellow double headed arrow). The infundibular sleeve is obviously discrete from the septal structures.
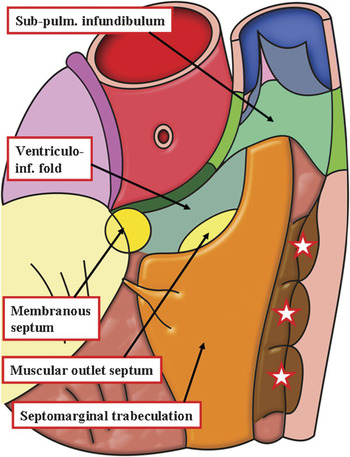
Figure 4 The cartoon shows the building blocks of the outflow tract of the normal right ventricle. It is impossible to distinguish the components representing the outlet septum and the ventriculo-infundibular fold in the normal situation without resorting to dissection (See Figs 1 and 2). The stars show the location of the septoparietal trabeculations.
The phenotypic features of tetralogy of Fallot
It is the divorce of the building blocks of the normal outflow tract, as shown in Figure 4, which provides the phenotypic feature for recognition of tetralogy of Fallot. The essence of the lesion is anterocephalad malalignment and deviation of the insertion of the muscular outlet septum relative to the limbs of the septomarginal trabeculation, coupled with an abnormal arrangement of the septoparietal trabeculations (Fig. 5).

Figure 5 The photograph, taken from the apex of the morphologically right ventricle looking towards the base, shows the phenotypic features of tetralogy of Fallot. Note that the septal attachment of the muscular outlet septum, which separates the overriding component of the aortic outlet from the subpulmonary infundibulum, and is marked by the star, is inserted antero-cephalad relative to the anterior limb limb of the septomarginal trabeculastion (yellow Y). The parietal extension of the outlet septum merges with the ventriculo-infundibular fold, which separates the leaflets of the aortic and tricuspid valves in the roof of the right ventricle.
These muscular structures are particularly well seen when the heart is sectioned to simulate the subcostal oblique echocardiographic cut (Fig. 6). Describing the building blocks in this fashion removes the confusion that has existed in the past concerning the nature of the structures producing subpulmonary obstruction. As can be seen in Figure 6, the obstruction is produced at the mouth of the infundibulum by the squeeze between the malaligned outlet septum and the septoparietal trabeculations. Problems with the use of septal band for description of these structures are revealed by study of a classic monograph devoted to tetralogy of Fallot.Reference Kirklin and Karp11 In illustrations of angiograms, it is the septoparietal trabeculations that are identified as the septal band. In contrast, it is the septomarginal trabeculation that is described in this fashion when referring to illustrations of autopsied hearts.

Figure 6 The heart has been sectioned to replicate the oblique subcostal echocardiographic cut, showing the phenotypic feature of tetralogy. The muscular outlet septum is inserted antero-cephalad to the limbs of the septomarginal trabeculation (yellow Y), with hypertrophy of the septoparietal trabeculations. The mouth of the subpulmonary infundibulum (yellow bracket) is squeezed between these components of the outflow tract. There is continuity between the leaflets of the aortic and tricuspid valves in the postero-inferior margin of the interventricular communication (star).
The outlet septum, of course, is exclusively a right ventricular structure in tetralogy. The ventricular septal defect is floored by the limbs of the septomarginal trabeculation, with the right ventricular origin of the overriding aortic valve supported by the ventriculo-infundibular fold (Fig. 4). These 3 anatomical features of the tetralogy, therefore, all reflect the abnormal location of the outlet septum. The outlet septum itself, nonetheless, can be markedly malaligned in antero-cephalad direction without there being subpulmonary stenosis, as in the so-called Eisenmenger defect (Fig. 7). It is the combination of the deviation of the outlet septum, together with the abnormal relationship relative to the septoparietal trabeculations, therefore, that produces the pathognomonic anatomy, as shown in Figure 8. The malaligned outlet septum continues to support a free-standing subpulmonary muscular infundibulum (Fig. 9). It is variation in the length of the subpulmonary infundibulum that underscores some of the anatomic variability within the lesion, along with other features such as the boundaries of the interventricular communication, and the extent to which the aortic valve is connected within the right ventricle.

Figure 7 The heart shown has been sectioned in the same plane as the one in Figure 6. There is again antero-cephalad deviation of the muscular outlet septum relative to the septomarginal trabeculation (yellow Y), but in this instance without producing subpulmonary obstruction (yellow bracket). This is the Eisenmenger defect. Note the lack of hypertrophy of the septoparietal trabeculations (stars).

Figure 8 The cartoon shows how the building blocks of the normal outflow tract (see Fig. 5) come apart one from the other in the setting of tetralogy of Fallot. The sub-pulmonary (sub-pulm.) obstruction is produced by a squeeze between the muscular outlet septum and the hypertrophied septoparietal trabeculations. Note the interventricular communication is cradled between the limbs of the septomarginal trabeculation, and in the variant shown is bordered postero-inferiorly by fibrous continuity between the leaflets of the aortic and tricuspid valves, this area incorporating the membranous septum (see text for further discussion).

Figure 9 The adjacent parts of the subaortic and subpulmonary outlets have been removed from a heart with tetralogy of Fallot and sectioned. The section shows how the narrowed subpulmonary infundibulum is made up of the outlet septum (star) and the free-standing infundibular sleeve (double headed arrow). There is an extensive tissue plane (dots) between the infundibulum and the aortic root, with marked off-setting of the leaflets of the arterial valves.
The interventricular communication
The hole between the ventricles is directly beneath the overriding aortic valvar orifice. It can thus be considered an outlet defect. The muscular outlet septum itself is usually well-formed, albeit malaligned relative to the rest of the muscular septum. Part of the pathognomonic anatomy of tetralogy, therefore, is that the outlet septum is a right ventricular, rather than an interventricular, structure (Fig. 6). When describing the margins of the ventricular septal defect, so as to remove any potential confusion, it is necessary to provide a definition of the plane of space nominated as the defect. When there is overriding of the aortic valve, any number of a series of planes within the cone of space subtended from the valvar attachments to the crest of the muscular ventricular septum can justifiably be nominated as the septal defect. Of these planes, 3 are particularly important (Fig. 10). The interventricular plane is the direct cranial continuation of the long axis of the ventricular septum, which extends to the undersurface of the aortic valvar leaflets during diastole, but reaches as far as the aortic arch when the aortic valve is open during ventricular systole. The plane from the leftward margin of the aortic valve to the crest of the ventricular septum represents the outflow tract from the left ventricle. During surgical repair, it is the plane extending from the rightward margin of the attachments of the aortic valve that is crucial, since this is the locus along which the surgeon will attach the patch used to reconnect the aorta with the left ventricle (Fig. 10). It is the margins of the cone of space as viewed from the right ventricle, therefore, which we define as the ventricular septal defect. This locus itself can then show significant anatomic variability depending on the specific inter-relationships of the building blocks of the outflow tract.

Figure 10 This heart from a patient with tetralogy of Fallot has been sectioned in “four chamber” plane to show the aorta riding the crest of the ventricular septum. There are 3 important planes within the cone of space subtended from the crest of the ventricular septum to the attachments of the overriding aortic valve. The green arrows mark the outlet from the left ventricle, whilst the blue dotted arrows show the continuation of the long axis of the muscular ventricular septum (blue solid line). It is the red arrows, however, that mark the plane that we describe as the interventricular communication, since this is the locus along which the surgeon will place the patch to reconnect the aorta to the left ventricle.
In about four-fifths of Caucasian patients, the ventriculo-infundibular fold stops short of the postero-inferior limb of the septomarginal trabeculation, permitting fibrous continuity to exist between the leaflets of the aortic and tricuspid valves (Fig. 5). In many instances, the area of fibrous continuity is reinforced by a flap representing the ventricular component of the membranous septum (Fig. 11). Defects of this type are directly comparable to typical perimembranous defects opening to the outlet of the right ventricle in the absence of subpulmonary obstruction (Compare Figs 6 and 7). In that the postero-inferior margin is an area of fibrous continuity between the leaflets of the aortic and tricuspid valves, the defect is unequivocally perimembranous. This means that, as in all other hearts with concordant atrioventricular connections, the guides to the location of the atrioventricular conduction axis are the apex of the triangle of Koch and the position of the medial papillary muscle (Fig. 11). As the axis penetrates through the central fibrous body, it is frequently overlaid by a remnant of the interventricular membranous septum, which may on occasions become aneurysmal. The septal remnant itself, called the membranous flap, is safe tissue for anchorage of sutures provided such stitches are placed with care. The flap is directly superficial to the penetrating bundle. Sutures placed deeply in this area, therefore, can produce complete heart block. It is probably safer to place sutures through the leaflet of the tricuspid valve, which usually overlaps the membranous flap in this area of the defect, albeit removed during the dissection of the heart shown in Figure 11. Once it has perforated, the non-branching atrioventricular bundle lies within the left ventricular part of the aortic outflow tract, but is almost always distant from the septal crest, the branching atrioventricular bundle typically staying remote from the septal crest. In a minority of hearts, the bundle may branch directly astride the septum. Such an arrangement places the bundle at risk should sutures be placed into the crest of the septum (Fig. 12).

Figure 11 The heart from a patient with tetralogy of Fallot and a perimembranous defect has been dissected so as to show the right ventricular margin of the ventricular septal defect. The postero-inferior margin is an area of fibrous continuity between the leaflets of the tricuspid, aortic, and mitral valves, and incorporates the remnant of the interventricular membranous septum (star). The rightward margin (green dotted line) is the ventriculo-infundibular fold, the roof is the muscular outlet septum (blue dotted line), and the leftward and anterior margin is the crest of the muscular ventricular septum (purple dotted line). The location of the atrioventricular conduction axis has been superimposed on the image, with the hatched oval showing the atrioventricular node, the yellow dotted line the axis on the left ventricular aspect of the septum, along with the left bundle branch (green dashed lines), and the red line showing the right bundle branch.

Figure 12 In this patient with tetralogy and a perimembranous septal defect, the surgeon placed the stitches to secure the patch through the crest of the ventricular septum, as was the vogue at that time. One of the stitches has produced an area of haemorrhage on the left ventricular aspect of the septal crest. As shown by the histological section (inset), the haemorrhage has infiltrated the non-branching atrioventricular bundle (red arrow in inset). The open arrow in the inset shows the bite of the suture through the septal crest.
The second most common variation, occurring in about one-fifth of Caucasian patients, is characterised by muscular continuity throughout the right ventricular margins of the defect. This is because the postero-caudal limb of the septomarginal trabeculation fuses with the ventriculo-infundibular fold. An intact membranous septum is then found between the muscular fold and the ventricular septal structures. With the atrioventricular conduction axis running postero-inferior relative to the membranous septum, the muscular fold, together with the membranous septum itself, separates the conduction tissues from the crest of the ventricular septum (Fig. 13). When the muscular fold is of good dimensions, as is usually the case, the entire muscular margins of the defect are suitable for anchorage of sutures, providing the stitches are not placed too deeply.

Figure 13 In this heart from a patient with tetralogy of Fallot, the postero-caudal limb of the septomarginal trabeculation (purple dotted line) fuses with the ventriculo-infindibular fold (green dotted line) in the postero-inferior margin of the defect. This protects the conduction axis (hatched oval, yellow dotted line, and red line). Note the hypertrophied septoparietal trabeculation (star) and the outlet septum (blue dotted line). When viewed from the right ventricle, all margins of the defect in this variant are made of muscle.
There is then yet a third variety of defect, characterised by presence of a fibrous rather than a muscular outlet septum. This is the doubly committed and juxta-arterial defect, by far the least common in the Western World, but commoner in the Far East and South America. The defect is both subaortic and subpulmonary as a consequence of failure of formation of a complete muscular subpulmonary infundibulum. Such defects can either be found with fibrous continuity between the leaflets of the aortic and tricuspid valves, making them also perimembranous (Fig. 14), or with a muscular postero-inferior rim, the latter being the most frequent finding. When it is present, the muscular postero-inferior border will protect the atrioventricular conduction axis, but in the heart shown in Figure 14, the conduction axis will be at direct risk in the postero-inferior margin of the defect.

Figure 14 In this heart from patient with tetralogy of Fallot, the ventricular septal defect is roofed by fibrous continuity between the leaflets of the arterial valves (blue dotted line), making it doubly committed, and also extends to an area of fibrous continuity between the aortic and tricuspid valves, the ventriculo-infundibular (green dotted line) fold stopping short of the postero-caudal limb of the septomarginal trabeculation (purple dotted line). Because the defect extends to become perimembranous, the conduction axis will be at potential risk in its postero-inferior margin. Note the remnant of the interventricular membranous septum, which forms the so-called membranous flap.
All patients with tetralogy will possess an interventricular communication with the features of one of the variations as described above. Such outlet defects, however, can also extend into the inlet component of the ventricle, either as the ventricular component of an atrioventricular septal defect, or in association with malalignment between the atrial and ventricular septal structures and straddling and overriding of the tricuspid valve. In the latter situation, the atrioventricular conduction axis arises from an anomalous atrioventricular node formed at the point of union between the malaligned ventricular septum and the right atrioventricular junction. The outlet defects can also co-exist with muscular inlet defects, the conduction axis then coursing through the muscular bar separating the holes.
Narrowing of the subpulmonary infundibulum
As we have explained, the subpulmonary stenosis, which is an essential part of tetralogy, is produced by the ‘squeeze’ between the anterocephalad malalignment of the outlet septum and the abnormal situated septoparietal trabeculations (Fig. 6). The septoparietal trabeculations, often additionally hypertrophied, although not universally so, extend onto the ventricular free wall. They can be removed by the surgeon without fear of causing damage to significant structures. In the past, it was also frequent to resect the parietal extension of the outlet septum, but with the shift to performing surgery in younger children, such destructive manoeuvres are often unnecessary. This can be an important point, since extensive resection of the musculature can result in infiltration of blood and oedema into the atrioventricular conduction axis, known to be a substrate for junctional ectopic tachycardia in the postoperative period. The maximal area of stenosis, when viewed from the apical part of the right ventricle, is seen as an obvious mouth to the subpulmonary infundibulum, the so-called “os infundibulum”. Additional stenosis can also be found more proximally within the ventricle, produced either by hypertrophy of the moderator band, which is one of the septoparietal trabeculations, or by prominent apical trabeculations. This gives the arrangement often described as “two-chambered right ventricle”. The subpulmonary infundibulum itself, distal to its mouth, varies markedly in length. In some instances, when the ventricular septal defect is doubly committed, the infundibulum is no longer an exclusively muscular structure (Fig. 14). In other instances, the narrowed infundibular chamber has considerable length (Fig. 15). There is a spectrum between these extremes, but measurements of series of hearts, when compared to measurements of normal hearts,Reference Becker, Connor and Anderson5, Reference Howell, Ho, Anderson and Elliott6 show that the infundibulum is longer in the setting of tetralogy. In addition of the muscular stenosis, it is also usual to find obstruction at valvar level, with the valve itself often possessing two rather than three leaflets. Further stenotic lesions can then be found within the pulmonary arterial pathways.

Figure 15 The heart from this patient with tetralogy of Fallot has been opened through the subpulmonary infundibulum, showing the considerable length of the infundibular chamber in this instance. There is a spectrum of length, but overall the infundibulum is longer than in the normal heart.
Overriding of the aortic valve
In the normal heart, although the right aortic sinus of the aortic valve overrides spatially the crest of the muscular ventricular septum, the leaflets of the valve are attached exclusively within the left ventricle. Whenever the ventricular septum is deficient, part of the circumference of the aortic valvar orifice becomes attached to, and supported by, right ventricular structures. Such aortic overriding is more obvious when the outlet septum is deviated so as to become exclusively a right ventricular structure, as in tetralogy of Fallot (Fig. 6), or the Eisenmenger ventricular septal defect (Fig. 7). The precise degree of override, in other words the proportion of the aortic valvar circumference supported by right as opposed to left ventricular structures, can vary between 5% and 100%. This feature has obvious surgical significance. A much larger patch will be required to re-connect the aorta to the left ventricle when the greater part of its circumference is supported by the right ventricle. This feature also has implications for nomenclature. When defined on the basis of connections, if more than half of the circumferences of both great arterial valves are connected in the same ventricle, then there is double outlet ventriculo-arterial connection. In the context of tetralogy of Fallot, therefore, if more than half of the leaflets of the aortic valve are hinged from right ventricular structures, the entity co-exists with the double outlet ventriculo-arterial connection.
Other lesions of the pulmonary circulation
Although the subpulmonary infundibulum is usually the narrowest part of the pulmonary outflow tract, other lesions are to be found elsewhere in the outflow tracts and the pulmonary arteries. Pulmonary valvar stenosis is a frequent accompaniment. This is sometimes due to domed stenosis, more frequently to stenosis of a bicuspid valve, or to stenosis of a valve with three leaflets. The valvar lesion is rarely the major cause of obstruction, albeit that in some young infants it can be the predominant finding. The valve can also become imperforate as an acquired change. So-called absence of the leaflets of the pulmonary valve is another important lesion. Most usually, the valve is represented by an annular array of fibrous rudiments, usually found with dilation of the pulmonary trunk and its branches (Fig. 16). Stenoses within the pulmonary arteries themselves are of major surgical significance, and usually occur at branching sites from the bifurcation outwards. Lack of origin of one pulmonary artery, typically the left, from the pulmonary trunk is by no means infrequent. The isolated pulmonary artery is almost always present, usually being connected by the arterial duct, or ligament, to some part of the system of aortic arches. Rarely, one pulmonary artery may arise directly from the ascending aorta, but then it tends to be the right one which is anomalously connected. Major systemic-to-pulmonary collateral arteries are sometimes present in association with tetralogy and pulmonary stenosis, but in association with normal right and left pulmonary arteries. Such arteries can be the sole source of pulmonary arterial flow when tetralogy co-exists with pulmonary atresia.

Figure 16 The illustration shows tetralogy of Fallot with so-called “absence” of the leaflets of the pulmonary valve. In reality, the leaflets form an annular rudimentary array at the ventriculo-arterial junction. Note the dilation of the pulmonary trunk and its branches, albeit that the ventriculo-arterial junction itself is narrowed.
Associated anomalies
Many other lesions can coexist with tetralogy. Patency of the oval foramen is common, and a deficiency of the floor of the oval fossa is far from infrequent. In addition to a second inlet muscular ventricular septal defect, straddling of the tricuspid valve, or presence of a common atrioventricular valve, features already emphasised, the most important associated lesion from the stance of the surgeon is probably anomalous origin of the anterior interventricular coronary artery from the right coronary artery. A right aortic arch, though not of functional importance, is also common. When detected, it alerts to the diagnosis of tetralogy. Aortic incompetence is commoner in older patients.
Conclusions
We have defined tetralogy of Fallot on the basis of antero-cephalad deviation of the outlet septum, or its fibrous remnant, relative to the limbs of the septomarginal trabeculation, combined with an abnormal relationship to the septoparietal trabeculations. This anatomic combination produces subpulmonary obstruction, and results also in presence of an interventricular communication, with part of the circumference of the aortic valve supported by right ventricular structures. The fourth part of the combination of lesions defined by Fallot himself, namely right ventricular hyperptrophy, is a haemodynamic consequence of the anatomic abnormality. We have emphasised the considerable variation in the anatomic features constituting the tetralogy, but have shown that all of these can be described in simple fashion by taking note of the locations and interrelationships of the building blocks of the normal right ventricular outflow tract. These features are just as obvious to the clinician using echocardiographic interrogation as to the morphologist working with the autopsied specimen. Concentration on these phenotypic features should, hopefully, resolve any ongoing disputes concerning description and nomenclature.


















