Many congenitally malformed hearts have been the subject of semantic disagreement. For most of these, advances over the last decade have resulted in resolution of the conflicts, with the realization that approaches to surgical treatment provide a means of grouping together entities previously considered morphologically discrete. The best example of that latter situation is the realization that arguments concerning allegedly univentricular hearts can be resolved by acknowledging the functionally univentricular nature of the circulation produced by the Fontan procedure.Reference Jacobs and Anderson1 A situation that still retains the potential to generate disagreement, however, is that in which both arterial trunks arise either entirely, or in their greater part, from the morphologically right ventricle. In the past, such double outlet from the right ventricle was considered a partial form of transposition, the argument being made that, in this setting, only the aorta was placed across the septum, the pulmonary trunk retaining its appropriate origin from the right ventricle.Reference Abbot2 And, at that time, when an anterior aorta was taken as the major criterion for transposition, it was frequent to describe patients as exhibiting double outlet with transposition.Reference Van Mierop3 Nowadays, most consider discordant atrioventricular connections, or alignments, to be the essence of transposition,Reference Van Praagh4 so most accept that it is better to distinguish this arrangement from that in which both arterial trunks arise from the right ventricle.Reference Jaggers, Cameron, Herlong and Ungerleider5 Debate continues, however, as to whether tetralogy of Fallot can co-exist with double outlet right ventricle,Reference Edwards6 and whether it is still necessary for patients to have infundibular musculature interposing between the leaflets of the atrioventricular valves and both arterial valves in order to make the diagnosis of double outlet.Reference Walters, Mavroudis, Tchervenkov, Jacobs, Lacour-Gayet and Jacobs7 As with the functionally univentricular heart, the surgical approach used for correction can serve to arbitrate these ongoing debates.Reference Walters, Mavroudis, Tchervenkov, Jacobs, Lacour-Gayet and Jacobs7, Reference Lacour-Gayet8 In this review, therefore, we discuss the anatomy of the hearts unified because both arterial trunks arise in their entirety, or in their greater part, from the morphologically right ventricle. We show how discussions of the anatomical features of the disparate hearts unified in this fashion can provide the basis for diagnosis during life, and serve to guide the most appropriate strategies for surgical correction.
How should we define double outlet right ventricle?
There is now overwhelming consensus that the optimal approach to diagnosis of patients with congenitally malformed hearts is to use a segmental approach.Reference Van Praagh9, Reference Anderson10 Irrespective of the words used to describe the various segments, it is also agreed that part and parcel of this approach is analysis of the fashion in which the components of the segments are joined together, with some describing this feature in terms of alignments,Reference Van Praagh9 and others accounting for the connections of the adjacent structures.Reference Anderson10 Again, irrespective of whether this feature is described in terms of alignments or connections, it is the way in which the arterial trunks are supported by the ventricular mass that is the basis of the entity described as double outlet right ventricle.Reference Walters, Mavroudis, Tchervenkov, Jacobs, Lacour-Gayet and Jacobs7 Markedly disparate phenotypes are unified within this grouping. Many of the hearts have infundibular sleeves supporting the leaflets of both the aortic and pulmonary valves within the right ventricle (Fig. 1). And, in most such examples, both arterial trunks unequivocally are supported in their entirety by the morphologically right ventricle (Fig. 1). Hearts with bilateral infundibular structures, nonetheless, can also exhibit overriding of the orifice of either the aortic (Fig. 2) or the pulmonary valve (Fig. 3). Bilateral infundibulums can also rarely be found in the setting of concordant or discordant ventriculo-arterial connections, or alignments. Thus, the presence of bilateral infundibulums does not guarantee the co-existence of double outlet right ventricle.

Figure 1 The heart shown has unequivocal double outlet right ventricle with subaortic interventricular communication and bilateral infundibulums.

Figure 2 In this specimen, the aorta is overriding the crest of the ventricular septum, with its greater part supported in the right ventricle, and there is a completely mucular subpulmonary infundibulum. There is pulmonary stenosis produced by a squeeze (red bracket) between the deviated outlet septum and anomalous septo-parietal trabeculations – the hallmark of tetralogy of Fallot, but with the ventriculo-arterial connection of double outlet right ventricle. The yellow Y shows the septomarginal trabeculation.

Figure 3 This is an example of the so-called Taussig-Bing malformation with overriding of the pulmonary trunk, but with double outlet ventriculo-arterial connections. The interventricular communication is still between the limbs of the septomarginal trabeculation (yellow Y) but opens in subpulmonary position because the muscular outlet septum inserts to the ventriculo-infundibular fold.
One of the most important principles established over the past few decades to guide the analysis of the congenitally malformed heart is the so-called “morphological method”.Reference Van Praagh, David, Wright and Van Praagh11 This principle states, quite rightly, that structures which are themselves variable should not be defined on the basis of another variable structure. It follows, therefore, that it is inappropriate to use infundibular morphology, known to be markedly variable, to define the fashion in which the arterial trunks are supported by the ventricular mass. As we will show, modern diagnostic techniques permit this feature of ventriculo-arterial connections, or alignments, to be determined with great accuracy. Examination of any large series of hearts grouped together because the arterial trunks arise in their greater part from the morphologically right ventricle reveals that a significant proportion exhibit fibrous continuity between the leaflets of one of the arterial valves and the atrioventricular valves (Fig. 4). It is most logical, therefore, to diagnose double outlet right ventricle whenever all of the circumferences of both arterial valves, or the greater part of both circumferences, are supported by the ventricular chamber possessing the apical trabecular component of morphologically right type.

Figure 4 The heart shown has double outlet ventriculo-arterial connection with subaortic interventricular communication, but this time with fibrous continuity between the leaflets of the aortic and mitral valves in the roof of the interventricular communication. The defect itself remains within the limbs of the septomarginal trabeculation (yellow Y).
Is double outlet right ventricle a diagnosis in its own right?
From the discussion above, it will be evident that double outlet, if defined as suggested, describes but a small part of the heart, namely a specific pattern at the ventriculo-arterial junctions. Hearts of many different types can fulfill the criterion for definition. Double outlet, therefore, is not a diagnosis in its own right, but a term with which to group hearts having the same ventriculo-arterial connection, or alignment. The hearts falling within the group can have any arrangement of the atrial appendages, or situs, and can be found with any atrioventricular connections, or alignments. When used in isolation, the term usually describes patients having usual atrial arrangement, or situs solitus, and concordant atrioventricular connections, or alignments. Even within this grouping, it is then possible to find multiple combinations of associated malformations, and further variation in terms of infundibular morphology, arterial interrelationships, and coronary arterial anatomy.Reference Wilcox, Ho, Macartney, Becker, Gerlis and Anderson12 Space does not permit us to define and describe all of these in details. Suffice it to say that, if necessary, all should be described. Of the associated malformations, the most important is the interventricular communication. This feature does deserve further discussion.
What is the interventricular communication?
We have chosen purposely to describe the hole between the ventricles, almost uniformly present when both arterial trunks arise from the right ventricle, as the interventricular communication rather than the ventricular septal defect. This is because there is a fundamental difference in the nature of this communication when the arterial trunks arise from the same ventricle as opposed to the more usual situation in which each arterial trunk arises from its own ventricle. Recognition of this difference serves also to distinguish those hearts with overriding arterial valves best considered as having double outlet right ventricle.
In the situation in which the arterial trunks arise from their own ventricle, be the ventriculo-arterial connections concordant or discordant, the so-called ventricular septal defect can be closed to reconstitute the ventricular septum (Fig. 5). In contrast, if we consider the situation in which both arterial trunks arise from the morphologically right ventricle, then of necessity the muscular outlet septum is exclusively a right ventricular structure (Fig. 6). The plane of space that must be closed so as to restore biventricular circulations is within the cavity of the right ventricle. It is not the interventricular communication. Indeed, in this setting, it would be a disaster to close the interventricular communication, which in essence is the outlet from the morphologically left ventricle. Instead, the interventricular communication must be tunneled to one or other of the arterial roots so as to restore biventricular circulations. Ideally this is to the aortic root, but in some circumstances it is tunneled to the pulmonary root. It is the feasibility of tunneling the interventricular communication to one or other of the arterial trunks that determines the options for surgical repair. Thus, in the situation of overriding of one or other arterial valve, if the surgeon considers that he or she has done no more than close the hole between the ventricles, it follows that initially the ventriculo-arterial connections will have been concordant or discordant. If, in contrast, the surgeon is of the opinion that the repair has required tunneling of the overriding valve to the left ventricle, then almost certainly the ventriculo-arterial connection was initially one of double outlet.

Figure 5 The cartoon shows how, in the setting of concordant or discordant ventriculo-arterial connections, the ventricular septal defect, or interventricular communication, can be closed by the surgeon so as to separate the pulmonary and systemic circulations. The star shows the crest of the muscular ventricular septum.

Figure 6 The cartoon shows the situation in which both arterial trunks arise from the right ventricle (compare with Fig. 4). In this instance, it would be a disaster for the surgeon to close the interventricular communication. The star again shows the crest of the muscular ventricular septum.
How do we distinguish double outlet connection in the setting of overriding arterial valves?
It is this problem that lies at the root of the controversy concerning the relationship between tetralogy of Fallot and double outlet right ventricle, and similarly between double outlet and discordant ventriculo-arterial connections when the pulmonary valve overrides a hole between the ventricles (Fig. 3). In either setting, the specific type of ventriculo-arterial connection does not depend on the phenotypic morphology, but rather on the proportion of the overriding valvar leaflets supported within the right rather than the left ventricle. This feature should be determined by assessing the short axis of the circumference of the valvar orifice to the cord subtended by the ventricular septum (Fig. 7), rather than on the plane of the long axis of the septum itself. The plane of the long axis of the septum can vary within the cardiac cycle relative to the overriding arterial root. Comment has often been made that the skilful echocardiographer can subjectively turn a patient with concordant connections into one with double outlet simply by varying the direction of the interrogating ultrasonic beam. This is not the case when the cord of the septum is assessed relative to the circumference of the valvar orifice. The new techniques now permitting three-dimensional reconstruction should permit these distinctions to be made with ever-increasing accuracy. This approach can diffuse the spurious arguments concerning the distinction of tetralogy and double outlet. The facts are that tetralogy is defined on the basis of phenotypic morphology, whereas double outlet describes no more than a ventriculo-arterial connection, or alignment. The two features can, and do, co-exist. It is also the case, of course, that surgical correction is likely to be much easier when double outlet is found in the setting of an overriding arterial valve, or rarely when both arterial valves override. This is simply because less tunneling will be required so as to reconnect the overriding valvar orifice to the morphologically left ventricle.

Figure 7 The cartoon shows how an overriding valve, in this instance the aortic valve, is assigned to one or other ventricle according to the location of the cord subtended by the plane of the ventricular septum relative to the overriding valvar orifice (blue dotted line). In this instance, the greater part is supported by the right ventricle (green surround), with the lesser part (yellow surround) remaining in the left ventricle. The effective ventriculo-arterial connection, therefore, is double outlet right ventricle.
How variable is the relationship of the interventricular communication to the arterial roots?
Having determined that both arterial trunks arise exclusively from the right ventricle, or in presence of overriding valves that the greater part of both arterial trunks is supported by the right ventricle, the most important task confronting the paediatric cardiologist is to determine the feasibility for surgical reconstruction so as to achieve biventricular circulations. The landmark investigation of Lev and his colleaguesReference Lev, Bharati, Meng, Liberthson, Paul and Idriss13 provided the basis for description of the interventricular communication. As they showed, the defect can be subaortic, subpulmonary, doubly committed, or non-committed. The defect itself does not move around the septum to produce these variations. In most instances, the defect is cradled within the limbs of the septomarginal trabeculation, or septal band (Figs 2–4). It is then the orientation of the muscular outlet septum, or its fibrous remnant, that determines whether the defect opens beneath the subaortic outlet, the subpulmonary outlet, or both outlets.Reference Hosseinpour, Jones, Barron, Brawn and Anderson14 It is also these patterns that produce the typical presentations of patients unified because of the double outlet ventriculo-arterial connection, namely those with the variants resembling tetralogy, transposition, and ventricular septal defect.Reference Walters, Mavroudis, Tchervenkov, Jacobs, Lacour-Gayet and Jacobs7 In these situations, surgical treatment is relatively straightforward. The results nowadays are uniformly excellent, with the caveat that the surgical tunneling procedure is likely to be more extensive when there are bilateral infundibular structures as opposed to overriding of an arterial valvar orifice, of fibrous continuity between an arterial and an atrioventricular valve. It is in patients with the so-called non-committed defects that surgical repair is most challenging. This situation is typically produced when the defect is no longer between the limbs of the septomarginal trabeculation, but is rather a perimembranous defect opening to the inlet of the right ventricle, often in the setting of a common atrioventricular junction, or a muscular defect in either the inlet or apical part of the ventricular septum (Fig. 8).Reference Stellin, Ho, Anderson, Zuberbuhler and Siewers15 In these circumstances, it may still be possible to achieve biventricular surgical repair.Reference Lacour-Gayet8 It is the task of the diagnostician to establish the feasibility of achieving construction of an interventricular tunnel in this setting.

Figure 8 In this heart with double outlet right ventricle and bilateral infundibulums, the interventricular communication is non-committed, because it is a muscular inlet defect. Note that, in this heart, the tension apparatus of the tricuspid valve interposes between the defect and both arterial outlets, making potential biventricular surgical repair most unlikely.
Echocardiographic approach to diagnosis
As with all congenitally malformed hearts, the echocardiographic approach to double outlet right ventricle requires a methodical sequential segmental approach. Recognition of double outlet right ventricle requires a complete understanding of the anatomic features of the ventriculo-infundibular fold, which must be distinguished from the outlet, or infundibular or conal, septum. Most importantly, the echocardiographer needs to ascribe the outlet septum as either a right ventricular or interventricular structure, this being the key to distinguishing double outlet right ventricle from hearts which have one-to-one ventriculo-arterial connections.
The task of identifying double outlet right ventricle by echocardiography is not always an easy one. Not uncommonly, echocardiographers, surgeons, and pathologists may disagree on the assignment of the great arteries to the respective ventricles. Depending upon the plane used for imaging, the arterial trunks may appear to be committed to different ventricles. For example, in a subcostal coronal plane, the aorta may appear to arise from the right ventricle, whereas in the parasternal short axis view, the aorta may appear to arise from the left ventricle. Some have attempted to simplify the process for the echocardiographer by stating categorically that, if there is mitral to aortic discontinuity, then the heart by definition represents double outlet right ventricle.Reference Van Praagh4 Such a classification can nearly always be made with a parasternal long-axis imaging plane. This approach can also result in misclassification of the ventriculo-arterial connections when, for example, the aorta arising predominantly from the left ventricle has its valve separated from the mitral valve by persisting infundibular musculature. The same problems arise when it is the pulmonary valve that is adjacent to the mitral valve. As has been discussed, therefore, we advocate assigning the arterial trunks to their respective ventricles based on the proportion of their valves committed to, and supported by, each ventricle. Hence, if greater than half of the circumference of the aortic valve is supported within the right ventricle, then the aorta is considered to have a right ventricular origin. As also discussed, the most appropriate way to make this assignation is through tomographic imaging in the short-axis plane.
Imaging in the subcostal coronal and sagittal planes are particularly helpful for defining the relationship of the atrioventricular and arterial valves. In particular, imaging in the subcostal coronal plane permits recognition of the ventriculo-infundibular fold (Fig. 9). If the fold is seen to be a right ventricular structure in this plane, then there is unlikely to be objections to classifying the heart as having double outlet right ventricle. For the purposes of anatomic classification, clinical management, and surgical repair, double outlet right ventricle has been classified based on the relationship of the interventricular communication to the arterial outlets. It is essential that the echocardiographer adequately image the building blocks of the outflow tracts so as correctly to describe this relationship.

Figure 9 The subcostal coronal view shows how the outlet muscular septum, separating the outlets to the aorta (AO) and the pulmonary trunk (PA), is exclusively a right ventricular structure.
Sub-aortic interventricular communication
This is the most common form of double outlet right ventricle, and two-thirds of patients fall into this category. The lesion is most commonly corrected surgically by creating a tunnel from the left ventricle to the aorta. Patients with this arrangement must be imaged in such a way to demonstrate to the surgeon the likely patency of the potential pathway from the left ventricle to the aorta. This is best achieved using subcostal planes of imaging, as the depth of field commonly allows the projection of the salient structures in a single sweep. The subcostal sagittal plane can also oftentimes display this relationship quite well. Some employ an additional subcostal imaging plane, namely the long-axial oblique plane, which is at an angle of 45° relative to the subcostal coronal and sagittal planes. The oblique plane is also very well suited for projecting the pathway from the left ventricle to the aorta. This view is particularly good at determining the distance between the interventricular communication and the aortic valve in those cases with a long ventriculo infundibular fold, and hence a substantial subaortic infundibulum. The parasternal long-axis view also provides an excellent plane with which to display the relationship of the interventricular communication to the arterial trunks (Fig. 10).Reference Smallhorn16 It can be difficult in this plane, however, to assign the commitment of arterial trunks to their respective ventricles, in other words to determine the precise degree of override. A parasternal sweep through the base of the heart generally provides a better perspective with which to determine the ventriculo-arterial alignments.

Figure 10 The parasternal long-axis image demonstrates total commitment of the aorta AO) to the right ventricle (RV). The interventricular communication (IVC), representing the outlet from the left ventricle (LV), is in subaortic position.
Once the echocardiographer has established the existence of double outlet right ventricle, and that the interventricular communication is subaortic, it is important to ensure that there is a potentially unobstructed pathway to the aorta. Hence, it is important to determine the size of the interventricular communication. In a small percentage of patients having double outlet right ventricle with subaortic interventricular communication, the defect itself is relatively small, and may be the site of subaortic obstruction should the surgeon create a baffle from the interventricular communication to the aorta without enlarging the hole between the ventricles (Fig. 11). Enlarging the interventricular communication is not without risk. The atrioventricular conduction axis is likely to be related to the postero-inferior rim, and injudicious resection can result in heart block. So as to identify correctly those subjects in which it is necessary to enlarge the interventricular communication, we suggest indexing its size to the diameter of the aortic valvar orifice. When the interventricular communication is less than four-fifths of the aortic valvar diameter, surgical resection should be considered.Reference Rychik, Jacobs and Norwood17 Another potential source of obstruction is the presence of straddling atrioventricular valves (Fig. 12). When the defect is subaortic, then it is the tricuspid valve which is likely to straddle. The presence of straddling does not preclude baffling the left ventricle to the aorta, but it makes the repair much more complex. In some cases, it may be possible to transect or re-implant cordal attachments. To aid the surgeon, therefore, it is important optimally to image the papillary muscles and cordal attachments of both atrioventricular valves in all patients with double outlet right ventricle.

Figure 11 The transesophageal image shows that the interventricular communication between the right (RV) and left (LV) ventricles is small relative to the size of the aorta (AO). This may be due in part to the presence of an additional muscular interventricular communication, not seen in this image.

Figure 12 The apical image demonstrates straddling of the tension apparatus of the mitral valve through the interventricular communication.
Another common feature of double outlet right ventricle with subaortic interventricular communication is the presence of subpulmonary stenosis. Significant subpulmonary stenosis occurs in approximately one-third of these patients. This is the essence of double outlet right ventricle of the tetralogy type. As with cases of tetralogy of Fallot with concordant ventriculo-arterial connections, it is important for the echocardiographer to identify several key components to aid surgical repair. The severity of the subpulmonary stenosis may aid in predicting the likelihood of developing hypercyanotic spells. The feature is best imaged in a subcostal sagittal or subcostal right axial oblique views, or in the parasternal short-axis view. These views show the antero-cephalad and leftward displacement of the outlet septum. Occasionally, the subpulmonary obstruction can be the result of muscle bundles within the right ventricle, so-called 2-chambered right ventricle. Pulmonary valvar hypoplasia can also occur in some settings. In order to relieve obstruction within the right ventricular outflow tract, the surgeon may need to perform an incision across the junction between the infundibulum and the pulmonary trunk, also-called transannular incision. Careful measurement of the diameter of the ventriculo-pulmonary junction in subcostal and parasternal planes is necessary to guide this decision. Many centres describe the size of the pulmonary valvar orifice using standard deviations, or Z-scores. A Z-score of less than −3.0 will predicate the need for a transjunctional patch. It is also important to identify any significant coronary arterial branches extending across the subpulmonary infundibulum, since an anomalous anterior interventricular artery, or a very prominent infundibular branch from the right coronary artery, may prevent complete resection of muscular subpulmonary stenosis, and hence necessitate insertion of a conduit from the right ventricle to the pulmonary arteries. The origins of the coronary arteries are best imaged in a parasternal short-axis view.
Post-operative assessment of the repair of double outlet right ventricle with sub-aortic interventricular communication should focus upon several keys components. Sub-aortic stenosis can occur immediately after repair, or develop in the intermediate period. The mechanisms can include a restrictive interventricular communication, or obstruction related to the geometry of the interventricular baffle. Transoesophageal post-operative imaging should include a detailed assessment of this pathway. If identified in the operating room, the surgeon can revise the interventricular baffle, or resect the margins of the interventricular communication as needed. Another post-operative complication is a residual interventricular communication. Such residual interventricular shunting most commonly occurs in or near the outlet septum (Fig. 13). These can be among the difficult residual lesions to identify and repair surgically. The echocardiographer must pay careful attention to the margins of the interventricular baffle that abut the outlet septum. Identification of residual subpulmonary or pulmonary obstruction is also vital. The echocardiogram may demonstrate the need for more aggressive resection, or indicate the need for a transjunctional patch.
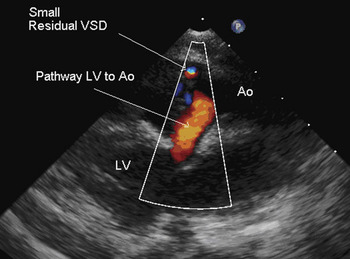
Figure 13 The parasternal long-axis view, with colour Doppler, demonstrating unobstructed flow from the left ventricle (LV) to the aorta (AO) through the surgically created baffle. There is a small residual ventricular septal defect (VSD) at the margins of the baffle.
Subpulmonary interventricular communication
The second most common form of double outlet right ventricle is that with a subpulmonary interventricular communication. As discussed, it is the variability in the arrangement and attachment of the right ventricular outlet septum that determines the commitment of the interventricular communication to the arterial trunks. When the defect is subpulmonary, the outlet septum is attached to the ventriculo-infundibular fold, rather than to the anterior limb of the septomarginal trabeculation and the underlying muscular ventricular septum. The eponym Taussig-Bing anomaly is often applied to this lesion. As when the interventricular communication is subaortic, the subpulmonary interventricular communication is imaged well using subcostal planes. The parasternal long-axis view reveals the potential patency of the pathway from the left ventricle to the pulmonary trunk required for creation of biventricular circulations (Fig. 14) The aorta tends to be remote from the interventricular communication when the defect is subpulmonary. The parasternal short-axis view shows how the anterior margin of the defect is formed by the junction of the anterior limb of the septomarginal trabeculation, the free wall of the right ventricle, and the pulmonary valve.
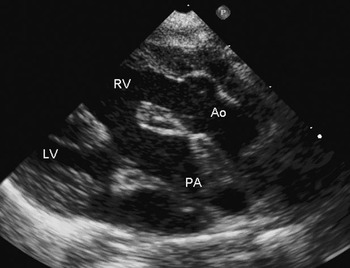
Figure 14 This parasternal long-axis view of a sub-pulmonary interventricular communication demonstrates the potential pathway from the left ventricle (LV) to the pulmonary trunk (PA). Other abbreviations: RV – right ventricle; AO – aorta.
There are number of subtle anatomic features in the setting of a subpulmonary interventricular communication that can have profound clinical implications. This is the type of lesion most frequently associated with straddling of the mitral valve, the valve itself often being cleft. There can also be subpulmonary or subaortic stenosis, the latter usually due to infundibular stenosis. All of these findings can complicate surgical repair. When the chosen approach is an arterial switch procedure, with baffling of the interventricular communication to the native pulmonary outflow tract, then native subpulmonary stenosis will become subaortic stenosis after the arterial switch. More common is native subaortic stenosis. A prominent right-sided ventriculo infundibular fold narrows the region of the anterior wall of the right ventricle. In some cases there is a fibromuscular element to the stenosis. This subaortic region is best imaged using the subcostal sagittal plane. The parasternal long-axis view can also demonstrate the fibromuscular elements. Subaortic stenosis is often associated with the presence of severe coarctation or interruption of the aortic arch (Fig. 15). Careful imaging of the aortic arch from suprasternal planes, therefore, is also crucial. As is the case with discordant ventriculo-arterial connections, identification and delineation of the origin and course of the coronary arteries is essential. Certain variations of coronary arterial anatomy are known to complicate the arterial switch, and must be identified if present (Fig. 16). The most important of these lesions is an intramural course, characterized by an echobright appearance of the coronary artery in close proximity to the zone of apposition between the aortic valvar leaflets. These features are best appreciated in the parasternal short-axis view.

Figure 15 This suprasternal sagittal image shows coarctation of the aorta (AO). The patient has double outlet right ventricle with a subpulmonary interventricular communication.

Figure 16 The parasternal short-axis view demonstrates origin of the circumflex coronary artery from the right (Rt) coronary artery in a patient with double outlet right ventricle and subpulmonary interventricular communication, the anterior (Ant) interventricular artery arising from the other facing aortic (Ao) valvar sinus PA – pulmonary trunk.
Post-operative echocardiographic assessment again requires attention to any residual interventricular communications, or obstruction of the interventricular communication creating functional subaortic stenosis. Complications related with coronary arterial implantation may result in segmental or global impairment in ventricular contractility. Supravalvar aortic or pulmonary stenosis can occur at the sites of anastomosis of the arterial trunks after the switch. And residual obstruction may occur in the aortic arch.
Doubly committed interventricular communication
In these variants, the roof of the common ventricular outlet is divided by a fibrous raphe separating the conjoined facing leaflets of the aortic and pulmonary valves. The floor of the defect is the crest of the ventricular septum. In some views, the large size of the interventricular communication gives the impression of double outlet left ventricle. Use of suitable tomographic planes, such as the subcostal coronal views, will determine whether the major portions of both arterial valves are supported within the right ventricle. (Fig. 17) The fibrous raphe can be deviated in some cases, resulting in subaortic or subpulmonary stenosis. The goal of echocardiography in these cases is to aid the construction of a suitable interventricular baffle. An understanding of anatomy is crucial preoperatively, as the conduction axis can be at great risk.

Figure 17 The subcostal coronal image (a) shows double outlet right ventricle with a doubly committed interventricular communication, the defect opening directly beneath the aorta (AO) and the pulmonary trunk (PA). Note the relatively deficient outlet septum. Other abbreviations: RV – right ventricle; LV – left ventricle. The parasternal long axis image (b) demonstrates a potential pathway from the left ventricle to the pulmonary trunk (PA), but imaging in the parasternal long axis (c) also shows a potential pathway from the left ventricle to the aorta (AO).
Non-committed interventricular communication
A less common form of double outlet right ventricle is the non-committed type. While some debate exists as to which hearts are worthy of this appellation, functionally this represents the defect in which an unobstructed pathway from the left ventricle to one of the arterial trunks can be created only with difficulty. In this setting, the subcostal coronal scan shows that the arterials roots have no direct relationship with the interventricular communication. The interventricular communication is typically no longer positioned between the limbs of the septomarginal trabeculation. Thus, the defect can be apical and muscular, or a perimembranous defect opening to the inlet of the right ventricle. These defects are rare, and have traditionally been managed by conversion to a functionally univentricular circulation. Some investigators also include as non-committed defects those in which the subarterial outlets are separated from the interventricular communication by the tension apparatus of the atrioventricular valves. In these settings, the subcostal coronal views show the interventricular communication committed to the antero-inferior aspect of the septum (Fig. 18). As the transducer is swept toward the base of the heart, the roof of the defect is seen to be formed by the tricuspid valve, or the bridging leaflets of a common atrioventricular valve (Fig. 19).

Figure 18 The subcostal coronal view shows a non-committed interventricular communication (arrow). The defect is located in the antero-inferior part of the muscular septum, and is distant from the origins of both the aorta (AO) and the pulmonary trunk (PA), although it would be possible surgically to create a pathway to the subpulmonary outflow tract. Other abbreviations: RA – right atrium; RV – right ventricle; S – septum.

Figure 19 The subcostal coronal image demonstrates the superior bridging leaflet in a patient with atrioventricular septal defect and common atrioventricular valve along with double outlet right ventricle (RV). Both the aorta (AO) and the pulmonary trunk (PA) arise exclusively from the right ventricle.
Repair of such lesions has historically been precluded by concern that an interventricular baffle will irrevocably damage the atrioventricular valves. These forms of double outlet right ventricle are commonly seen in the setting of isomerism of the atrial appendages, particularly right isomerism, and when the interventricular communication is part of an atrioventricular septal defect. For the echocardiographer, it is important to recognize that the non-committed interventricular communication presents an enormous technical challenge to the surgeon. Careful identification of the atrioventricular valves and the papillary muscles is critically important.
In recent years, there has been burgeoning interest in creating complex intracardiac baffles so as to tunnel the left ventricle to the aortic or pulmonary valve even when the defect, at first sight, seems to be non-committed.Reference Serraf, Lacour-Gayet and Houyel18 This approach may involve the use of two separate patches. In such cases, it is imperative that the echocardiographer be familiar with the surgical approach, and be vigilant in the postoperative period in searching for residual interventricular shunts, as well as any obstruction in the surgically created pathway. When there is straddling of atrioventricular valvar tension apparatus, the post-operative study must also ensure the maintenance of adequate atrioventricular valvar function.
Other complicating features
Since double outlet right ventricle describes only the ventriculo-arterial connection, or alignment, there can be a wide spectrum of associated lesions. The lesion can occur in the setting of isomerism of the atrial appendages, absent atrioventricular connections, tricuspid and mitral valvar abnormalities, atrioventricular septal defects, anomalies of the systemic and pulmonary veins, and lesions of the great arteries. As with all congenitally malformed hearts, therefore, the echocardiographer must undertake a segmental analysis of the cardiac anatomy to account fully for the other lesions that might occur. The echocardiographer should apply the same nomenclature for these other variants. When double outlet right ventricle occurs in the setting of a functionally univentricular heart, it is particularly important to give consideration to the location of the outlet septum, and the potential for subaortic or subpulmonary obstruction.
Surgical approach to the double outlet right ventricle
The surgical approach varies among the sub-types of double outlet right ventricle, and even within the sub-types, based on the size of the interventricular communication, and the relationship of the papillary muscles to the proposed interventricular pathway. In some cases, palliative surgery involving creation of a systemic-to-pulmonary shunt may be the best option. In other cases, managing in functionally univentricular fashion may provide the best chance for survival over the intermediate term.
Sub-aortic interventricular communication
These patients often develop significant congestive heart failure on the basis of a large left-to-right shunt. As such, surgical repair is often undertaken in the first several months of life. If there is significant sub-pulmonary stenosis, the circulation may be more balanced, and surgery undertaken later in the first year of life.
The interventricular communication can be closed either through the right atrium or through a right ventriculotomy.Reference Bradley, Karamlou and Kulik19 A single patch is sufficient to baffle adequately the left ventricle to the aorta in nearly all cases. When there is significant sub-pulmonary stensosis, the surgical repair is similar to elective repair of tetralogy of Fallot.Reference Macartney, Rigby, Anderson, Stark and Silverman20 As with tetralogy of Fallot, surgical repair may require a transjunctional incision adequately to relieve subpulmonary muscular obstruction. While there is some controversy in determining when the pulmonary valve can be spared, many surgeons assess both valvar morphology and diameter. In general, a Z-score of less than −2.5 should be adequate. When the valvar leaflets are thickened or dysplastic, a transjunctional incision may be required even when the diameter of the valvar orifice is deemed adequate. It may also be necessary to enlarge the interventricular communication in a small proportion of patients. Echocardiographic measurements can aid in making this decision. Another useful rule of thumb is to consider surgical enlargement of the interventricular communication when the diameter of the defect is less than the diameter of the aortic valvar orifice plus 2 millimetres subsequent to the onset of cardioplegia.Reference Serraf, Lacour-Gayet and Houyel18 Resection of the interventricular communication must be achieved without injuring the atrioventricular conduction axis. In general, sutures should be placed on the right ventricular side of the patch, using the leaflets of the tricuspid valve when necessary.
Subpulmonary interventricular communication
Though infants with this physiology can be managed medically for weeks to months, most centres prefer to undertake a complete repair in the neonatal period. The repair consists of baffling the interventricular communication to the native pulmonary trunk, and performing the arterial switch procedure. As is the case when the ventriculo-arterial connections are discordant, coronary arterial anomalies are frequent. A complex intramural course may preclude coronary arterial reimplantation.Reference Baslaim21 In such cases, a Rastelli-type repair may be employed, with baffling of the left ventricle to both aortic and pulmonary valves, and placement of a conduit from the right ventricle to the pulmonary trunk. As obstruction within the aortic arch is frequent, surgical relief may be needed. This can be achieved using an end-to-end anastomosis, transverse enlargement of the arch with a homograft, or in some cases repair of an interrupted arch. It was necessary to resection part of the subaortic infundibulum in two-fifths of patients in one reported series.Reference Lacour-Gayet8
Doubly committed interventricular communication
This lesion accounts for less than one-twentieth of all children with double outlet right ventricle undergoing surgical intervention. The surgical repair can be complicated by the size and geometric orientation of the interventricular communication relative to the great arteries. As is the case with other forms of doubly committed ventricular septal defects, the superior margin of the patch needs to be secured to the fibrous raphe between the arterial valves, usually favouring the pulmonary side. Intraventricular rerouting can be carried out via incisions to the right atrium and the pulmonary trunk. To ensure reconstruction of an unobstructed pulmonary pathway, a limited right ventriculotomy may be required in some patients.Reference Uemura, Yagihara, Kadohama, Kawahira and Yoshikawa22
Non-committed interventricular communication
This lesion presents one of the greatest challenges for surgical repair. Because both great vessels are remote from the interventricular communication, creating an unobstructed interventricular is complex. In most patients, the left ventricle can be baffled to the aorta. Under certain circumstances, it may be more feasible to baffle to the pulmonary trunk and perform an arterial switch procedure. Due to the challenges of creating a geometrically complex pathway, there may be an advantage to delaying complete repair until later infancy. Banding of the pulmonary trunk may function as a temporizing measure in such cases. If the interventricular communication is located in the muscular septum, the risk of obstruction may be greater than for other forms of double outlet right ventricle. Enlargement was needed in more than half of one series.Reference Belli, Serraf and Lacour-Gayet23 This procedure is usually undertaken via a right venticulotomy, and more than one patch may be required to avoid injuring the subvalvar apparatus of the tricuspid valve.Reference Barbero-Marcial, Tamanati and Jatene24
Satisfactory results can undoubtedly be produced by complete repair. There are, nonetheless, certain factors that preclude successful separation of the pulmonary and systemic circulations. If the interventricular communication is located in the inlet septum, and the defect is small, enlargement may not be feasible. Although short-term outcomes are encouraging, longitudinal follow-up is warranted by those using this strategy. Previous analysis of subjects with double outlet right ventricle showed that biventricular surgical repair had a much higher need for reintervention than a strategy of functionally univentricular palliation.Reference Bradley, Karamlou and Kulik19
Summary
Double outlet right ventricle represents a spectrum of congenitally malformed hearts in which the circumferences of both arterial valves, or the greater part of both circumferences, are supported by the right ventricle. Sub-classification based on the relationship of the interventricular communication to the great arteries provides a value framework for clinical management. Echocardiography remains the definitive modality for diagnosing this entity. As the appropriate surgical approach depends on both the relationship of the interventricular communication to the great arteries and the associated lesions, it is imperative that the echocardiographer and surgeon carefully review all anatomic features. Multiple imaging planes are needed to demonstrate how the surgeon can create an unobstructed pathway from the left ventricle to the great arteries. In some circumstances, the only reasonable option may be to consider the patient with double outlet right ventricle as possessing a functionally univentricular heart.





















