Anatomy of the normal mitral valve
Like the right ventricle,3 the left ventricle is best analyzed in terms of its inlet, apical trabecular, and outlet components. The mitral valve occupies the left ventricular inlet, with its skirt of leaflet tissue suspended from the annulus at the level of the atrioventricular junction, and extending to be attached to the papillary muscles. The insertions of the papillary muscles mark the junction between the inlet and apical ventricular components. Although orthogonal sections are required to demonstrate the entire arrangement, the essence of the valvar complex is well seen in the planes which replicate the parasternal long axis echocardiographic sections (Fig. 1).
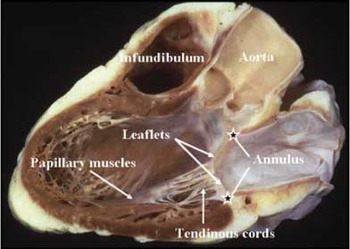
Figure 1. This section of a normal heart is cut to replicate the parasternal long axis section. It shows well the components of the mitral valvar complex. Note that the superior component of the annulus is a fibrous structure, formed from the area of fibrous continuity between the leaflets of the aortic and mitral valves (see Figure 2).
It is an easy matter in such sections to recognize the annulus, an integral part of the atrioventricular junction, the leaflets, the tendinous cords, the papillary muscles, and their ventricular support.2 Attention to the parasternal long axis sections also emphasizes a fundamental difference between the normal mitral and tricuspid valves. Whilst the leaflets of the tricuspid valve are suspended almost exclusively from the muscular atrioventricular junctions,3 this is not the case for the mitral valve, since the atrioventricular and arterial valves of the left ventricle overlap within the ventricular short axis, the roof of the ventricle being formed by the extensive area of fibrous continuity between the leaflets of the mitral and aortic valves (Fig. 2).

Figure 2. This section through a normal heart is cut in the short axis of the ventricular mass, and is viewed from the apex looking towards the base. It shows well the resemblance of the left atrioventricular valve to the Episcopal mitre, and demonstrates the solitary zone of apposition (dotted line) between the two leaflets of the valve. Note that the line of closure is oblique relative to the septum, with the papillary muscles positioned inferiorly and laterally within the left ventricle.
As a consequence, part of the annulus of the mitral valve, which is in continuity with the aortic root, is unrelated directly to an atrioventricular junction.
As we have already discussed, the mitral valve was named because of its perceived likeness to the episcopal mitre. Its alternative name, of course, was the bicuspid valve, thus distinguishing it from the atrioventricular valve of the right heart with its three leaflets. Examination of the pattern of closure of the valve confirms its bifoliate arrangement, albeit showing that the two leaflets are markedly dissimilar in their extent and structure (Figs. 1 and 2). One leaflet guards one-third of the circumference of the left atrioventricular junction, and is deep. The other guards two-thirds of the circumference, but is much shallower, typically possessing three subunits, known as scallops, along its length. Although Vesalius described the leaflets as being aortic and mural, a precedent which we are happy to follow, it is more usual to describe them as anterior and posterior. The alternative designations are attitudinally appropriate, which is more than can be said for the usual descriptions for the papillary muscles. These are usually described as being postero-medial and antero-lateral. In reality, they are positioned inferiorly and laterally (Fig. 2). At any event, a more important anatomic point is that a valve with two leaflets can have only one zone of apposition between them. The mitral valve, therefore, has only one commissure. This also extends from inferiorly to laterally within the atrioventricular junction, running obliquely relative to the short axis of the left ventricle. The tendinous cords from both sides of the ends of adjacent leaflets are attached to each of the paired papillary muscles, the muscles being positioned directly beneath the ends of the zone of apposition so as to act with maximal mechanical efficiency. In the past, various complicated systems have been used to categorize the tendinous cords connecting the leaflets of the valves to the papillary muscles and the ventricular muscle. Suffice it to say that, in the normal valve, all parts of the leaflets usually have cords connecting the free edge to the papillary muscles. Fan-shaped cords are found at the ends of the zone of apposition between the leaflets, but also between the scallops of the mural leaflet. Some of the cords, rather than attaching to the free edge, insert on the ventricular aspect of the aortic leaflet. These are called the strut cords, their attachments producing the layered arrangement of the aortic leaflet emphasized by Leonardo da Vinci. Other shorter cords, the basal cords, run directly from the undersurface of the mural leaflet to the parietal ventricular wall.
Analysis of the congenitally malformed mitral valve
As we have already emphasized, the concept of the valvar complex2 also proves it worth when used to underscore the analysis of the congenitally malformed mitral valve. Thus, lesions can involve primarily the leaflets, the tendinous cords, or the papillary muscles. In many circumstances, all components of the valve are involved, as is typically the case in hypoplastic left heart syndrome. In this setting, when the mitral valve is stenotic, then typically the pathology involves leaf lets, tendinous cords, and papillary muscles. On rare occasions, however, the mitral valve can be miniaturized in the setting of hypoplasia of the left heart but not intrinsically stenotic. When the aortic valve is similarly miniaturized but not intrinsically stenotic, this sets the scene for potential biventricular repair.4 The mitral valve can also be malformed at several levels in the so-called Shone's syndrome.5
Even when the mitral valve is globally abnormal, the key to analysis is to recognize the extent of involvement of the various components. The leaflets can prolapse, or can be rendered stenotic by a fibrous shelf adherent to their atrial surface. Although this latter lesion is often termed “supravalvar” stenosis, this is a misnomer, since the stenotic lesion is usually an integral part of the leaflets. Indeed, this lesion should be distinguished from the supravalvar fibrous shelf found within the vestibule of the left atrium. A particularly significant lesion of the aortic or anterior leaflet is when it is cleft, the space between the components then pointing, of necessity, directly into the subaortic outflow tract (Fig. 3).

Figure 3. This anatomic specimen demonstrates a cleft of the aortic, or anterior, leaflet of the morphologically mitral valve (arrow). The atrioventricular septal structures are intact, albeit that there is a ventricular septal defect (asterisk). This cleft in the midpart of the aortic leaflet should not be confused with the zone of apposition between the left ventricular components of the bridging leaflets seen in hearts with deficient atrioventricular septation and common atrioventricular junction.
Such clefts are often seen in the setting of discordant ventriculo-arterial connections with a subpulmonary ventricular septal defect, the so-called Taussig-Bing malformation.6 The otherwise normally formed mitral valve, however, can exhibit a cleft in its aortic leaflet. This lesion should not be confused with the zones of apposition between the left ventricular components of the bridging leaflets in patients with common atrioventricular junction, since the left part of the common valve can never be converted surgically into a mitral valve, even if there is a separate valvar orifice exclusively committed to the left ventricle.7 The echocardiographer will readily recognize the difference by identifying the common as opposed to the left atrioventricular junction. In comparable fashion, it is important to distinguish dual orifices of the mitral valve, another lesion involving the valvar leaflets, from dual orifice of the left valve in the setting of common atrioventricular junction. The basic lesion producing dual orifices is a bridge of tissue joining adjacent leaflets,7 but each orifice can also; on occasion possess its own tendinous cords, attaching the orifices to separate papillary muscles.
The most obvious lesion involving the tendinous cords is straddling of the tension apparatus. Usually coexisting with overriding of the valvar annulus, the mitral valve straddles across the antero-superior part of the ventricular septum, and is typically seen, as with the cleft valve, in the setting of the Taussig-Bing malformation.6 It is part of a spectrum leading to double inlet right ventricle.7 Other lesions, such as the arcade lesion, also involve primarily the tendinous cords. This anomaly reflects abnormal fenestration of the ventricular support during development,8 the papillary muscles supporting the entire free edge of the leaflet along the arcade.
The major lesion involving the papillary muscles is the so-called parachute malformation, this also being an integral part of the Shone complex.5 As emphasized, in the normal heart the tendinous cords are shared between the paired papillary muscles, which are positioned inferiorly or laterally. In the parachute arrangement, all the tendinous cords insert to only one papillary muscle. This can either be because one muscle is deficient or absent, or because the two muscles are fused together to form a solitary mass. The latter variant is more likely to be found when the valve itself is more globally malformed.
Echocardiographic manifestations
The normal valve, and acquired mitral valvar disease
Echocardiography is the most useful noninvasive test for evaluating suspected mitral valvar disease. Transthoracic or transoesophageal cross-sectional imaging usually reveals its etiology, and whether there is evidence of underlying dilated cardiomyopathy, which can significantly disturb the function of the mitral valve. Colour and spectral Doppler evaluation is used to estimate the degree of regurgitation or stenosis. Echocardiography is also useful in assessing the size of the left atrial and ventricular chambers, and in evaluating left ventricular systolic function. These assessments carry important implications with respect to the timing of any surgery required to treat an abnormal valve.
The echocardiographer undertaking comprehensive analysis of the mitral valve, therefore, will always take care to assess
- The size and shape of the annulus.
- The mobility of the leaflets, in particular whether they prolapse, or show flail or restricted motion. This assessment will also include exclusion of thickening calcification, myxomatous degeneration, clefts, fusion along zones of apposition, perforations, vegetations, or abnormal shelves or membranes.
- The length and thickness of the tendinous cords, whether they are fused or ruptured.
- The number, structure, and function of the papillary muscles.
- Whether the function of the left ventricle is normal, globally deranged, or shows evidence of regional abnormalities of motion of the walls.
Detailed description of acquired mitral valvar diseases is beyond the scope of this review, but perhaps one abnormality that deserves brief attention is prolapse of the leaflets of the mitral valve. This is one of the most common, but also one of the most over-diagnosed, lesions of the mitral valve. It is best diagnosed by cross-sectional echocardiography, and exists when there is displacement of one or both leaflets across the plane of the atrioventricular junction by 2 millimetres or more during late systole, or by 3 millimetres or more if the prolapse is holosystolic.
Because the annulus of the valve is known to be saddle shaped, the normal leaflets can appear to prolapse in certain echocardiographic views, most notably in the apical two- and four-chamber views. The diagnosis, therefore, should be based primarily on the long-axis view (Fig. 4).
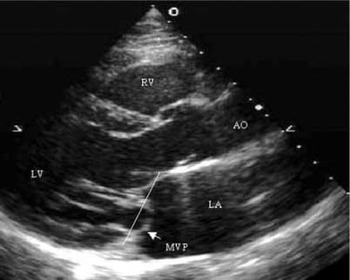
Figure 4. This parasternal long axis section shows the mural, or posterior, leaflet of the mitral valve prolapsing (MVP) beyond the plane of the atrioventricular junction. Abbreviations: AO: aorta; RV: right ventricle; LV: left ventricle; LA: left atrium.
In patients confirmed to have prolapsing valvar leaflets, echocardiography is also useful in determining the presence and severity of mitral regurgitation, assessing the size of the left atrium and ventricle, assessing left ventricular function, and establishing whether the prolapsing leaflets are also thickened or redundant.
Congenital malformations of the mitral valve
Echocardiographic examination of the mitral valvar complex must include not only delineation of the anatomy, and any variations from normal as discussed above, but also a detailed haemodynamic assessment of valvar function. From the functional as opposed to the strictly anatomical stance, we can identify three broad categories of abnormality, namely stenosis and hypoplasia, regurgitation, and inappropriate alignment of the valve and its tension apparatus. As already discussed, inappropriate alignment becomes manifest as override of the atrioventricular junction, and straddling of the tension apparatus (Fig. 5). Of necessity, this means there must be a ventricular septal defect, which typically opens to the outlet of the right ventricle Echocardiography is unsurpassed in delineating the cordal support of the atrioventricular valves.9, 10 Careful definition of the cords, and their supporting papillary muscles, therefore, must be a part of every echocardiographic examination in patients with ventricular septal defects, particularly to diagnose any straddling of the tension apparatus. When straddling is identified, it should be established whether the abnormal cords insert within the right ventricular cavity, on the right ventricular aspect of the septal surface, or on the crest of the ventricular septum. The proportion of valvar support derived from the abnormal cords should also be described.

Figure 5. The apical four-chamber echocardiographic image demonstrates minimal straddling of the tension apparatus of the mitral valve. The dashed arrow indicates the anomalous cords, which attach to the right ventricular surface close to the crest of the ventricular septum (asterisk) in this patient, who has double outlet right ventricle. This allowed surgical septation, with placement of the patch (arrowhead) farther to the right than usual, leaving the straddling cords, and part of the malaligned septum (asterisk), to the left. Abbreviations: L: left; LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle; S: superior.
Mitral valvar stenosis occurs when the functional orifice of the valvar complex is too small to allow free forward flow. This is often the consequence of a combination of abnormalities at multiple anatomic levels. For simplicity of presentation, we will describe the causes of stenosis separately, while recognizing the possibility of combined lesions, typically seen in the setting of the Shone complex. A common cause of obstruction is overall hypoplasia of the valve. When the degree of hypoplasia is mild, or even moderate, it is the related abnormalities affecting the patient and their valvar complex that will determine the clinical approach and outcome. When hypoplasia is the only valvar abnormality, manoeuvres designed to increase the flow across the valve, such as closing an atrial septal defect, or repairing anomalous pulmonary venous connections, will usually result in “catch up” growth of the valvar complex. The most convenient echocardiographic measurement of hypoplasia is the mitral annular z-score.11 Although the annular diameter can be measured in multiple planes, the most reproducible and convenient is the apical four chamber view. Malformations that result in congenital mitral obstructions include the stenosing supravalvar ring, congenital stenosis of the valvar leaflets, the arcade lesion, and the so-called “parachute” malformation.
The so-called “supravalvar” mitral ring is a circumferential ridge, typically on the atrial aspect of the valvar leaflets (Fig. 6). The ring restricts the diastolic excursion of the leaflets, reducing the functional orifice of the mitral inlet. Other abnormalities are present in almost all cases of supravalvar ring. Parachute deformity of the mitral valve is the most common co-existing lesion, seen in more than four-fifths of patients.12 On occasion, however, the ring can truly be found in supravalvar location, at the level of the atrioventricular junction. Not to be confused with division of the left atrium by an oblique fibromuscular shelf, as seen in classical “cor triatriatum”, this supravalvar ring is found at the level of the atrial vestibule (Fig. 7).

Figure 6. This long axis echocardiographic image shows the essence of the so-called “supravalvar” mitral ring (arrowheads). The ring is found downstream from the atrioventricular junction (asterisks), but proximal to the tips of the valvar leaflets. The circumferential shelf restricts the motion of the leaflets. This particular patient also had aortic coarctation, mild subaortic stenosis, a single papillary muscle, and a ventricular septal defect, in other words, Shone's complex.5 Abbreviations: A: anterior, LA: left atrium; LV: left ventricle; RV: right ventricle; S: superior.

Figure 7. These transesophageal two-chamber images show the more proximal form of supravalvar stenosing ring, where the abnormal shelf is attached at the level of the vestibule at the left atrioventricular junction (arrows, left hand panel). Note that unlike classical “cor triatriatum” the entire left atrium is upstream from this ring. Colour Doppler interrogation (right hand panel) shows diastolic acceleration and a narrow flow stream across the inflow tract consistent with important obstruction. Abbreviations: LA: left atrium; LV: left ventricle.
Congenital mitral stenosis also usually involves an abnormality of the mitral valvar leaflets, albeit that often there is co-existing annular hypoplasia, and abnormal positioning of the supporting papillary muscles. The valvar leaflets are usually thickened, and their diastolic motion is reduced. This results in a “reversed” doming in diastole, producing a stenotic orifice, with aliasing of flow detected at the tips of the leaflets (Fig. 8). The papillary muscles are often crowded within the left ventricular cavity, further reducing the effective opening of the valvar complex. Congenital subvalvar mitral stenosis, also described as the arcade or hammock lesion, is a consequence of thickening of the tendinous cords, combined with a reduction of the intercordal spaces. The abnormalities of the cords decrease the excursion of the leaflets, making distinction between the arcade lesion and congenital stenosis difficult at times. Indeed, on occasion the two abnormalities can co-exist. The compromise of the intercordal spaces, nonetheless, produces a unique appearance when the mitral inlet is interrogated using color Doppler. In most other stenotic lesions, flow enters the ventricle as a single central stream. In the setting of a mitral arcade, flow remains laminar throughout the mitral funnel from the annulus to the tips of the leaflets, but then divides into multiple small streams at high velocities of flow. This is due to the streams being deflected from the centre line of the valvar orifice, passing through the intercordal spaces and entering the ventricle at varying angles relative to the cords (Fig. 9). Parachute malformation of the mitral valve occurs when all (or most) cordal support is committed to a single papillary muscle (Fig. 10). The solitary muscle is often in the inferior position, but may also be found at the level of the mid-cavity, between the expected normal positions of the papillary muscles. The presence of a solitary zone of muscular support for all of the tendinous cords reduces the functional diastolic orifice of the valvar complex, but in the absence of other abnormalities, the resulting stenosis is usually not severe.

Figure 8. The long axis echocardiographic images reveal the presence of congenital mitral valvar stenosis. There is diastolic doming of the aortic leaflet of the valve (arrowhead in the upper left panel), and the tendinous cords are foreshortened. Colour Doppler mapping (middle panel) shows the disturbance of flow to begin at the tips of the leaflets (arrows). Abbreviations: A: anterior; LA: left atrium; LV: left ventricle; RV: right ventricle; S: superior.

Figure 9. These echocardiographic images were obtained from a patient with an obstructive mitral arcade. The valvar leaflets are relatively uninvolved, the major problem being thickening and dysplasia of the tendinous cords (dashed arrow – upper panel). This obstruction distal to the tips of the leaflets produces an unusual colour Doppler pattern of left ventricular inflow. There are multiple jets of diastolic colour, seen in the lower panel. These streams are angulated away from the usual direct path of flow between the left atrium and ventricle (solid arrows). The jets represent the flow through the reduced intercordal spaces, this phenomenon shifting the functional valvar orifice from the tips of the leaflets to the sub-valvar level. Abbreviations: L: left; LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle; S: superior.
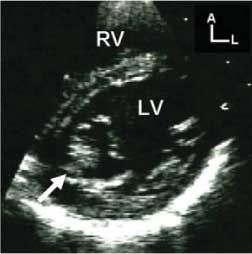
Figure 10. Images in this patient revealed a single, inferior left ventricular papillary muscle. This parasternal short axis scan was taken at cordal level, and demonstrates that the tendinous cords are completely committed to this inferior papillary muscle (white arrow). Abbreviations: A: anterior; L: left; LV: left ventricle; RV: right ventricle.
As we have emphasized throughout our review, congenital malformations that produce obstruction in the circulation through the left heart often co-exist. The most obvious example of this is Shone's complex.5 In its full-blown form, this consists of four levels of obstruction, namely a stenosing supravalvar mitral ring, parachute deformity of the mitral valve, subaortic stenosis, and coarctation of the aorta. It can be difficult to assess the haemodynamic importance of the varying lesions found in such close proximity. Gradients will become unreliable in the presence of decompressing shunts, and the most severe lesion may mask the importance of the others. A systematic and segmental approach to these patients, therefore, is mandatory. The echocardiographer must rely even more heavily than usual on the anatomic findings that relate to severity of stenosis in these cases.
Once the anatomic features of the abnormal valvar complex have been delineated, the functional status of the inlet must be determined. Doppler echocardiography has become the central technique used to supplement cross-sectional scanning in the assessment of valvar function. Although calculation of functional area of the valve is the gold standard for stratifying severity in mature patients with acquired stenosis, it is not reliable in young patients with a congenitally malformed valvar complex.13 It is the mean trans-valvar Doppler gradient, therefore, which should be used in most cases to grade the severity of obstruction. Since gradients are proportional to flow, one must always assess the Doppler data obtained in relation to the stroke volume crossing the mitral inlet. In the patient with an intact atrial septum, and a normal index of pulmonary flow, most consider a mean diastolic mitral gradient of less than 8 millimetres of mercury as representing mild stenosis, gradients between 8 and 12 millimetres as moderate, and larger gradients as severe. Haemodynamics assessed during exercise can often be helpful in determining the clinical significance of the stenosis. This is particularly true when the cause of symptoms is not immediately clear, as with a patient who also has a respiratory disorder. Increased trans-mitral stroke volumes, as in cases with combined stenosis and regurgitation, will exaggerate the gradient. Here the echocardiographer needs mentally to increase the gradient threshold for each degree of stenosis, and be certain that the degree of anatomic compromise in diastole is consistent with the final impression of the obstruction. Obviously, no set cut-points can be described for these patients, since each case will be relatively unique. When there is the potential for decompression through a moderate or large atrial septal defect, or the volume of flow to the lungs is reduced, Doppler gradients will understate the severity of stenosis, and may even be absent. In these patients, we must rely on careful anatomic definition of the valvar complex as the only method available for assessing the degree of stenosis.
Congenital mitral malformations that result in predominantly regurgitation are uncommon. Mitral regurgitation, if encountered, is most often acquired as a consequence of myocardial dysfunction secondary to other congenital cardiovascular malformations. Cardiomyopathies, both dilated and hypertrophic (Fig. 11), and inflammatory disorders, such as rheumatic heart disease, can also result in important regurgitation. In cases of hypertrophic cardiomyopathy where the valvar complex is not permanently deformed, relief of the dynamic outflow obstruction will often eliminate the regurgitation without any direct intervention on the valve itself.14
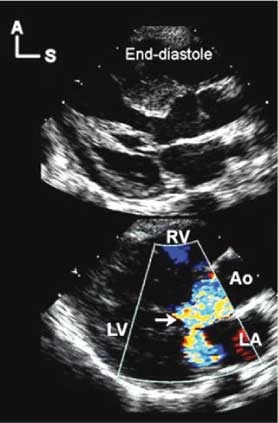
Figure 11. These parasternal long axis echocardiographic images were taken in a patient with hypertrophic obstructive cardiomyopathy. The mitral regurgitation seen in the lower Doppler image is acquired due to the influence of the obstruction in the left ventricular outflow tract on the mitral valvar complex. The image in the upper panel is taken in late diastole, and shows the tendinous cords positioned centrally within the ventricular cavity. As systole and the obstruction progress, the cords are drawn anteriorly into the outflow tract, ultimately contacting the septum at peak systole. As shown in the lower panel, such systolic distortion typically results in an eccentric and posteriorly directed jet of regurgitation. In most cases, relief of the obstruction will eliminate the regurgitation. Abbreviations: A: anterior; Ao: aorta; LA: left atrium; LV: left ventricle; RV: right ventricle; S: superior.
The neonate with a congenitally malformed mitral valve and predominantly regurgitation, but not stenosis, will often have shortened tendinous cords. This malformation is very difficult to treat. Many of these newborns will not survive, and those who do frequently require surgical replacement of the valve. Other congenital abnormalities associated with regurgitation are the isolated cleft, and the valve with dual orifices. As we have already emphasized in our section devoted to anatomy, the isolated cleft should be distinguished from the zone of apposition between the bridging leaflets as seen in the setting of atrioventricular septal defect with common atrioventricular junction, but is readily diagnosed using cross-sectional echocardiography (Fig. 12). Dual orifices in the mitral valve can be of comparable or unequal size. They are best recognized in short axis scans (Fig. 13). In diastole, the atrioventricular junction opens as two circles divided by the central bridge of valvar tissue, rather than in the expected ellipse of the normal valve. Treatment of the regurgitant valve with dual orifices must be individualized, being based on the anatomic cause of the insufficiency. When one of the orifices is associated with an anterior cleft, closure of the cleft will usually improve the situation. If only one orifice is involved, complete closure of that orifice can be considered, recognizing that some degree of stenosis, albeit often only mild, will result.

Figure 12. This short axis image shows an isolated cleft of the aortic leaflet of the mitral valve in a patient who also has a ventricular septal defect. The arrow points to the cleft, and the asterisk is within the ventricular septal defect. Abbreviations: A: anterior; L: left; LV: left ventricle; RV: right ventricle.

Figure 13. The parasternal short axis image demonstrates dual orifices within the mitral valve. The scan is taken at the level of the tendinous chords. The lateral orifice is marked by a black arrow. It is of moderate size in the example shown. Abbreviations: LV: left ventricle; RV: right ventricle.
Conclusions
As can be appreciated from our discussions, congenital abnormalities of the mitral valvar complex encompass the entire spectrum of valvar malformations. Attitudinally appropriate display of the structures involved facilitates both understanding of, and communication about, these malformations. Detailed descriptions of the anatomy and functional state of the valvar complex are essential to the planning and implementation of treatment for the patients affected by these malformations.
Acknowledgements
Robert H. Anderson is supported by grants from the British Heart Foundation together with the Joseph Levy Foundation. Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from R&D funding received from the NHS Executive.















