Anatomical variations of the aortic arch branching pattern may represent a high degree of variety depending on the origin and number of vascular structures arising from the aortic arch. The most common structure is called the “normal pattern”.Reference Thomson 1 , Reference Adachi 2 The variations of aortic arch branching are usually asymptomatic and detected incidentally by radiological examinations performed for other causes. However, supra-aortic vascular variations, particularly an aberrant subclavian artery, may present with symptoms such as dyspnoea and dysphagia resulting from tracheoesophageal compression.Reference Donnelly, Fleck, Pacharn, Ziegler, Fricke and Cotton 3 , Reference Alper, Akgun and Kantarci 4 Furthermore, detection of these variations is important in terms of preventing complications associated with surgery or endovascular interventional procedures of the aorta and its branches as well as thoracic or head and neck surgeries.
Determination of some aortic arch branching variations by conventional catheter angiography, a two-dimensional imaging modality, may be difficult because of superposition of the other large branches. Recently, multi-detector computed tomographic angiography has become the most important imaging modality for imaging of the aorta and its branches.Reference Türkvatan, Büyükbayraktar, Olçer and Cumhur 5 , Reference Lee, Siegel, Hildebolt, Gutierrez, Bhalla and Fallah 6 Compared with spiral computed tomography, multi-detector computed tomographic angiography offers higher quality two-dimensional and three-dimensional images as it has better properties such as a shorter acquisition time, narrower collimation, and increased temporal and spatial resolution.Reference Türkvatan, Büyükbayraktar, Olçer and Cumhur 5 , Reference Lee, Siegel, Hildebolt, Gutierrez, Bhalla and Fallah 6 However, it also has some disadvantages such as exposure to ionising radiation and use of iodinated contrast agents. Magnetic resonance angiography is an alternative non-invasive imaging method free of ionising radiation. Nonetheless, the spatial resolution of magnetic resonance is lower than that of multi-detector computed tomography.Reference Hellinger, Daubert, Lee and Epelman 7
Many studies focusing on determining the incidence of variation of the aortic arch branching pattern in the general population have been carried out using autopsy or conventional catheter angiography. The rates reported in the literature for these variations vary within a wide range (2.6–49%).Reference Thomson 1 , Reference Adachi 2 , Reference Williams, Aff, Schmeckebier, Edmonds and Graul 8 – Reference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 The number of multi-detector computed tomographic angiography studies on this subject is limitedReference Berko, Jain, Godelman, Stein, Ghosh and Haramati 16 – Reference Müller, Schmitz and Pauls 18 and most of them are case reports.
In this study, we aimed to investigate the frequency and gender distribution of variations of the aortic arch branching pattern by retrospectively reviewing the multi-detector computed tomographic angiography images of 1000 patients. Furthermore, by reviewing the present literature on this issue, we compared the results of our study with those of the others and discussed the clinical significance of these variations.
Materials and methods
Patient population
Between January, 2010 and September, 2011, a total of 1043 patients underwent thoracic computed tomographic angiography because of various reasons. A total of 32 patients who had aortic arch aneurysm/dissection, history of aortic arch surgery, or obstructive vascular disease in the supra-aortic branches as well as five patients in whom computed tomographic angiography yielded suboptimal results for identification of the aortic arch anatomy were excluded from the study. Only patients who have a normal left-sided aortic arch were included in this study. Therefore, three patients with a right-sided aortic arch and an aberrant right subclavian artery, one patient with a right-sided aortic arch and mirror-image branching, one patient with a right-sided interrupted aortic arch and an aberrant right subclavian artery, and one patient with a double aortic arch were all excluded from the study. In total, 1000 patients (610 male and 390 female; mean age: 56 years; age range: 17–94 years) were included in the study. Informed consent was obtained from all patients, and the study was approved by the local ethics committee of our hospital.
Computed tomographic angiography scanning protocol
A 64-detector computed tomography scanner (Aquilion, Toshiba Medical Systems, Tokyo, Japan) as well as the same protocol were used for examination of all patients. An area from the neck to diaphragm level was recognised as the scan field. The optimal scan time was determined using the automatic bolus tracking method (Sure Start, Toshiba Medical Systems). The region of interest was placed over the descending aorta, and an adjustment was made to ensure that the scanning would automatically start when the maximum contrast reached 180 HU. Thereafter, 80–100 ml of a non-ionic iodine contrast agent was administered at a rate of 4–5 ml/s, followed by delivery of 40 ml of saline using an automatic injector. A non-ionic iodine contrast agent (Iodixanol, Visipaque 320 mgI/ml; GE Healthcare, Milwaukee, Wisconsin, United States of America; or Iopromid, Ultravist 370 mgI/ml; Schering AG, Berlin, Germany) was used as an intravenous contrast material. The multi-detector computed tomography scan parameters were as follows: tube voltage, 120 kV; tube current, 200–440 mAs; collimation, 64 × 0.5 mm; gantry rotation time, 0.5 s; slice thickness, 1 mm; and slice interval, 1 mm.
Analysis of multi-detector computed tomography images
Computed tomographic angiography images of the 1000 patients were evaluated for variations in the aortic arch branching pattern. The variations in the branching of the aortic arch were categorised into seven types. The normal branching pattern of the aortic arch was defined as type 1. It consisted of three branches: the brachiocephalic trunk, which gives off two branches – the right subclavian and right common carotid arteries – the left common carotid artery; and the left subclavian artery. The brachiocephalic trunk, together with the left common carotid artery arising from the aortic arch in a common trunk – the “bovine” aortic arch – was defined as type 2. It consisted of a common trunk giving off the right subclavian artery, the right common carotid artery, and the left common carotid artery; and the left subclavian artery. The left vertebral artery arising directly from the aortic arch was defined as type 3. It consisted of the brachiocephalic trunk; the left common carotid artery; the left vertebral artery; and the left subclavian artery. The coexistence of type 2 and type 3 was defined as type 4. It consisted of the right subclavian artery, the right common carotid artery, and the left common carotid artery originating from a common trunk; the left vertebral artery; and the left subclavian artery. The right subclavian artery originating from the aortic arch as the last branch and reaching the right side by following a course anterior or posterior to the trachea and/or oesophagus – “aberrant right subclavian artery” – was called type 5. It consisted of the right common carotid artery, the left common carotid artery, the left subclavian artery, and the aberrant right subclavian artery. The coexistence of a bicarotid trunk, comprising carotid arteries arising from the aortic arch in a common trunk, and the aberrant right subclavian artery, comprising the bicarotid trunk, the left subclavian artery, and the aberrant right subclavian artery, was defined as type 6. Existence of an additional artery originating from the aortic arch, for example, thyroida ima artery comprising of brachiocephalic trunk, left common carotid artery, thyroida ima artery, and left subclavian artery, was defined as type 7.
Statistical analysis
The study data were analysed using the SPSS 14.0 (SPSS Corp., Chicago, Illinois, United States of America) statistical package program. The χ 2-test was used for investigating the association between gender differences and variations in the aortic arch branching pattern. A p-value < 0.05 was considered statistically significant.
Results
Of the 1000 patients, 792 (79.2%) had a normal aortic arch branching pattern – type 1 – and 208 (20.8%) patients had a variations in aortic arch branching (Fig 1). Among these 208 patients, 141 (14.1%) had a type 2 variation (Fig 2), 41 (4.1%) had a type 3 variation (Fig 3), 12 (1.2%) had a type 4 variation (Fig 4), six (0.6%) had a type 5 variation (Fig 5), seven (0.7%) had a type 6 variation (Fig 6), and one (0.1%) had a type 7 variation (Fig 7).

Figure 1 Type 1 variation of the aortic arch. A coronal volume rendered multi-detector computed tomography image showing normal branching pattern of the aortic arch giving rise to three branches: the brachiocephalic trunk (BT), which then branches into the right common carotid artery (RCA) and the right subclavian artery (RSA); the left common carotid artery (LCA); and the left subclavian artery (LSA). Ao = aorta.

Figure 2 Type 2 variation of the aortic arch. A coronal volume rendered multi-detector computed tomography image showing a common origin of the brachiocephalic trunk (BT) and the left common carotid artery (LCA). Ao = aorta; LSA = left subclavian artery; RCA = right common carotid artery; RSA = right subclavian artery.
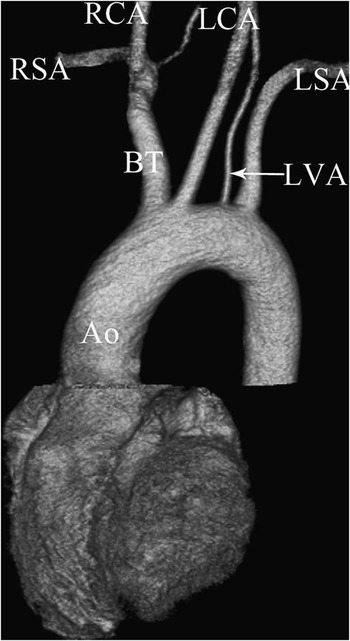
Figure 3 Type 3 variation of the aortic arch. A coronal volume rendered multi-detector computed tomography image showing the left vertebral artery (LVA) arising directly from the aortic arch. Ao = aorta; BT = brachiocephalic trunk; LCA = left common carotid artery; LSA = left subclavian artery; RCA = right common carotid artery; RSA = right subclavian artery.

Figure 4 Type 4 variation of the aortic arch. A posterior coronal volume rendered multi-detector computed tomography image showing a common origin of the brachiocephalic trunk (BT) and the left common carotid artery (LCA) and the left vertebral artery (LVA) arising directly from the aortic arch. Ao = aorta; LSA = left subclavian artery; RCA = right common carotid artery; RSA = right subclavian artery.

Figure 5 Type 5 variation of the aortic arch. A posterior coronal volume rendered multi-detector computed tomography image showing an aberrant right subclavian artery (ARSA). Ao = aorta; LCA = left common carotid artery; LSA = left subclavian artery; RCA = right common carotid artery.
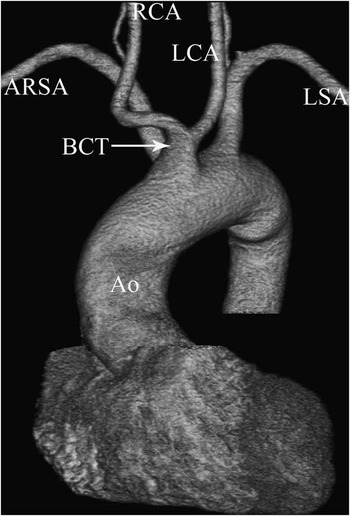
Figure 6 Type 6 variation of the aortic arch. An anterior coronal volume rendered multi-detector computed tomography image showing a common origin of the carotid arteries and an aberrant right subclavian artery (ARSA). Ao = aorta; BCT = bicarotid trunk; LCA = left common carotid artery; LSA = left subclavian artery; RCA = right common carotid artery.

Figure 7 Type 7 variation of the aortic arch. A coronal volume rendered ( a ) and oblique multi-planar reformatted ( b ) multi-detector computed tomography images showing a thyroidea ima artery (TIA) arising directly from the aortic arch. Ao = aorta; BT = brachiocephalic trunk; LCA = left common carotid artery; LSA = left subclavian artery; LVA = left vertebral artery; RCA = right common carotid artery; RSA = right subclavian artery; RVA = right vertebral artery; T = thyroid gland.
When variations in the aortic arch branching pattern were analysed with respect to gender; 488 (80%) of 610 males had a normal branching pattern and 122 (20%) had variations, whereas 304 (77.9%) of 390 females had a normal branching pattern and 86 (22.1%) had variations. Type 2 variation was observed in 86 (14.1%) males and 55 (14.1%) females, whereas a type 3 variation was observed in 25 (4.1%) males and 16 (4.1%) females. Type 4 variation was determined in seven (1.1%) males and five (1.3%) females. The incidences of type 5 variations among males and females were 0.3% (n = 2) and 1% (n = 4), respectively. Type 6 variation was observed in one (0.2%) male and six (1.5%) females, whereas a type 7 variation was observed only in one male (0.2%). In general, type 5 and type 6 variations were observed to be more common among females compared with males, and all the other types of variations were found to have similar incidences in both genders. The χ 2-test revealed no statistically significant correlation between the presence of supra-aortic vascular variations and gender differences (p = 0.165). The frequency and gender distribution of variations in the aortic arch branching pattern are shown in Table 1.
Table 1 The frequency and gender distribution of variations in the aortic arch branching pattern.

ARSA = aberrant right subclavian artery; BCT = bicarotid trunk; BT = brachiocephalic trunk; LCA = left common carotid artery; LSA = left subclavian artery; LVA = left vertebral artery; RCA = right common carotid artery; TIA = thyroida ima artery
Discussion
Anatomical variations seen in aortic arch branching are generally asymptomatic and diagnosed incidentally. However, variations leading to compression of the trachea and oesophagus may cause clinically significant symptoms. A typical example of such an event is an aberrant right subclavian artery, which may cause compression to the oesophagus and trachea during its retroesophageal and retrotracheal course.Reference Donnelly, Fleck, Pacharn, Ziegler, Fricke and Cotton 3 , Reference Alper, Akgun and Kantarci 4 At present, diagnosis of supra-aortic variations before interventional procedures is importance in terms of preventing complications that may occur during the procedures. In the thoracic endovascular graft replacement procedure, misinformation about the supra-aortic branching pattern may lead to an endoleak or ischaemic complications of the brain and the upper extremities. Diagnosis of supra-aortic vascular variations before surgical procedures involving the aorta and its branches, as well as thoracic or head and neck operations, has great importance.
The incidence of variation in the aortic arch branching pattern in the general population has been investigated by a low number of studies comprising post-mortem examinations, and many of these studies include a limited number of cases. The incidence of a normal aortic arch branching pattern has been reported as 82.4% by Thomson,Reference Thomson 1 whose study included 500 English cadavers, and as 83.3% by Adachi,Reference Adachi 2 whose study included 516 Japanese cadavers. Liechty et alReference Liechty, Shields and Anson 10 performed the largest post-mortem study on this subject in 1957; they performed a series of autopsies on 1000 cadavers and reported an incidence of a normal aortic arch branching pattern of 64.9%. In general, the incidence of a normal aortic arch branching pattern is reported to vary between 64.9% and 97.4% among Caucasians in post-mortem studies.Reference Thomson 1 , Reference Adachi 2 , Reference Williams, Aff, Schmeckebier, Edmonds and Graul 8 – Reference Nayak, Pai, Prabhu, D'Costa and Shetty 14 However, its incidence is known to be lower (51%) among black people. Natsis et alReference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 conducted a study using conventional catheter angiography images of 633 Greek patients and reported an incidence of a normal aortic arch branching pattern of 83%. Berko et alReference Berko, Jain, Godelman, Stein, Ghosh and Haramati 16 carried out a study by applying a retrospective review of the computed tomographic angiography images of 1000 patients among whom the incidence of a normal aortic arch was 65.9%; they succeeded in determining the ethnic origin of 72.7% of patients in the study population and observed that 50% of them were black people. Another study using computed tomographic angiography was carried out by Jakanani and AdairReference Jakanani and Adair 17 ; they studied 861 patients whose ethnic origins were unknown and reported an incidence of a normal aortic arch branching pattern of 74%. Another study carried out by Müller et alReference Müller, Schmitz and Pauls 18 by retrospective reviewing of contrast-enhanced computed tomography images of 2033 patients reported a frequency of normal aortic arch branching of 86.7%. In our study on 1000 patients, all of whom were Turkish, the incidence of a normal aortic arch branching was 79.2%. This rate was rate within the reported range for Caucasians (64.9–94.7%).Reference Thomson 1 , Reference Adachi 2 , Reference Williams, Aff, Schmeckebier, Edmonds and Graul 8 – Reference Nayak, Pai, Prabhu, D'Costa and Shetty 14
The incidence of a bovine aortic arch – type 2 variation – has a wide range reported in the literature (0.9–27.4%).Reference Thomson 1 , Reference Adachi 2 , Reference Williams, Aff, Schmeckebier, Edmonds and Graul 8 – Reference Liechty, Shields and Anson 10 , Reference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 The prevalence for black people is observed to be 41.4–45.6%.Reference Williams, Aff, Schmeckebier, Edmonds and Graul 8 , Reference McDonald and Anson 9 Although the incidence of a bovine aortic arch was found to be high by Berko et alReference Berko, Jain, Godelman, Stein, Ghosh and Haramati 16 (27.4%), it was found to be low by Nelson and SparksReference Nelson and Sparks 13 (1.03%) and Nizankowski et alReference Nizankowski, Rajchel and Ziolkowski 11 (0.9%). In our study, the incidence of a bovine aortic arch was 14.1%, which was a rate similar to that found by Natsis et alReference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 (15%). This variation generally presents with an asymptomatic status; however, it may rarely cause clinical symptoms. Furthermore, the presence of this variation may cause technical difficulties during stenting of the carotid artery and may eventually lead to neurological complications.Reference Faggioli, Ferri and Freyrie 19
A left vertebral artery originating directly from the aortic arch was recognised as a type 3 variation of aortic arch branching in our study. The incidence of this variation varies between 0.79% and 6.1% in the literature. Berko et alReference Berko, Jain, Godelman, Stein, Ghosh and Haramati 16 found that the incidence of a type 3 variation was 6.1%, whereas it was observed to be 2.5% by Lietchty et al.Reference Liechty, Shields and Anson 10 Furthermore, the incidence of type 3 variations was found to be as low as 0.79% in the conventional angiographic study by Natsis et al.Reference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 The reason behind such a low value may be the difficulty in detection of this variant artery with a small diameter because of possible vascular superpositions during conventional catheter angiography. Nizankowski et alReference Nizankowski, Rajchel and Ziolkowski 11 and Nelson and SparksReference Nelson and Sparks 13 observed that a type 3 variation was the second most common variation, in contrast to a bovine aortic arch. The incidence of this variation in our study was 4.1% and was within the range reported in the literature. It has been observed that a left vertebral artery originating directly from the aortic arch is not associated with a clinical symptom as long as there is no aneurysm in this artery. However, there are findings suggestive of the fact that the risk of spontaneous vertebral artery dissection is higher in such cases.Reference Dudich, Bhadelia and Srinivasan 20 The reason behind this tendency may be related to congenital structural defects in the arterial wall or changes in cerebral haemodynamics. Particularly during neurovascular interventional or surgical procedures, being unaware of this variation may lead to permanent neurological deficits due to damaging of the vertebral artery.Reference Daentzer, Deinsberger and Böker 21 Furthermore, in the presence of this variation, the patient may be misdiagnosed with a left vertebral artery occlusion.
In our study, a type 4 variation was defined as coexistence of the brachiocephalic and left common carotid arteries arising from the aortic arch in a common trunk and the left vertebral artery arising directly from the aortic arch. This variation, generally reported as case reports in the literature, has not been defined in post-mortem and angiographic studies with a large sample size. In the computed tomographic angiography study by Berko et al,Reference Berko, Jain, Godelman, Stein, Ghosh and Haramati 16 the incidence of this variation was 1.6%, whereas Jakanani and AdairReference Jakanani and Adair 17 reported an incidence of 2%. In our study, the incidence of a type 4 variation was 1.2%.
Among the variations in the aortic arch branching pattern, an aberrant right subclavian artery is the variant most likely to cause clinical symptoms. After originating from the aortic arch as the last branch, an aberrant right subclavian artery usually shows a retroesophageal and retrotracheal course and may require surgical treatment because it becomes tortuous and ectatic, particularly among the elderly, and induces compression on the oesophagus and trachea with serious symptoms. Diagnosis of this variation is important because, although rarely, it may be associated with other congenital cardiovascular abnormalities or recurrent laryngeal nerve abnormalities.Reference Kobayashi, Yuta, Okamoto and Majima 22 Furthermore, if right arm vessels are to be used for reaching the aorta as in conventional angiography, being unaware of such a variation may complicate the catheterisation process. In our study, a type 5 variation was defined as the presence of an aberrant right subclavian artery, whereas a type 6 variation was defined as coexistence of a bicarotid trunk – carotid arteries arising from the aortic arch in a common trunk – and an aberrant right subclavian artery. In the computed tomographic angiography study by Berko et al,Reference Berko, Jain, Godelman, Stein, Ghosh and Haramati 16 the incidence of an aberrant right subclavian artery alone was 0.8%, whereas the incidence of a coexistence of the bicarotid trunk and an aberrant right subclavian artery was 0.4%. In their conventional angiographic studies, Natsis et alReference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 did not detect an isolated presence of an aberrant right subclavian artery. In their study, the incidences of both an isolated bicarotid trunk and coexistence of a bicarotid trunk and an aberrant right subclavian artery were the same (0.16%). In our study population, the incidences of type 5 and type 6 variations were 0.6% and 0.7%, respectively, and no patient had an isolated bicarotid trunk. The incidences reported in the literature for an aberrant right subclavian artery and a combination of a bicarotid trunk and an aberrant right subclavian artery vary between 0.4–2% and 0.16–1.6%, respectivelyReference Nayak, Pai, Prabhu, D'Costa and Shetty 14 – Reference Jakanani and Adair 17 ; the findings of our study, 0.6% and 0.7%, respectively, were within the reported ranges. Generally, a bicarotid trunk is seen in combination with an aberrant right subclavian artery. Being aware of the presence of this condition, which is usually asymptomatic, is important because it may cause dyspnoea secondary to tracheal compression and may be associated with congenital abnormalities such as DiGeorge syndrome, oesophageal atresia, tracheoesophageal fistula, origin of the left coronary from the pulmonary artery, congenital polyvalvular disease, trisomy 13, trisomy 18, trisomy 21, tetralogy of Fallot, or a Noonan phenotype.Reference Wells, Landing and Shankle 23
The incidence of a thyroidea ima artery in the general population varies between 0.4% and 10%.Reference Thomson 1 , Reference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 Although this artery may originate from the common carotid, internal thoracic, pericardiophrenic, subclavian, thyrocervical trunk, inferior thyroid, or transverse scapular arteries, it may rarely originate directly from the aortic arch as well.Reference Tohno, Tohno and Matsumoto 24 Diagnosis of the presence of this artery is important for preventing possible complications that may arise during neck surgeries such as thyroid resectomy or laryngeal transplantation. Natsis et alReference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 reported an incidence of a thyroidea ima artery originating directly from the aortic arch of 0.16%. Similarly, we found an incidence of this variation – type 7 – in our study population of 0.1%.
Other variations in aortic arch branching patterns are very rarely seen. One of such variations is termed a “bilateral brachiocephalic trunk” – avian form – which is defined as two branches arising from the aortic arch, common origin of the carotid arteries, and common origin of the subclavian arteries. The incidence of this variation has been found to be 1.2–2% in post-mortem studies,Reference Liechty, Shields and Anson 10 , Reference Gupta and Sodhi 25 0.16% in the conventional angiography study by Natsis et al,Reference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 and 0.2% in the computed tomographic angiography study by Berko et al.Reference Berko, Jain, Godelman, Stein, Ghosh and Haramati 16 The “absence of brachiocephalic trunk” – bilateral carotid and subclavian arteries directly originating from the aortic arch – is another rare variation. Natsis et alReference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 reported an incidence of this variation, which is thought to have no clinical significance, of 0.16%. In our study on 1000 patients, there was no case of a bilateral brachiocephalic trunk or of a brachiocephalic trunk.
The incidence of variations in aortic arch branching with respect to racial differences has been found to be 48.3% in Afro-Americans, 33.1% in white Americans,Reference McDonald and Anson 9 23% in Indians,Reference Nayak, Pai, Prabhu, D'Costa and Shetty 14 18% in Portuguese,Reference Grande, Costa, Pereira and Aguas 12 17.6% in English,Reference Thomson 1 17% in Greeks,Reference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 16.7% in Japanese,Reference Adachi 2 16% in Koreans,Reference Shin, Chung, Shin, Im, Hwang and Kim 26 11.06% in Asians,Reference Piyavisetpat, Thaksinawisut and Tumkosit 27 and 2.6% in Polish individuals.Reference Nizankowski, Rajchel and Ziolkowski 11 In our study population that was entirely comprised of Turkish people, the incidence of a variation was 20.8%, which was similar to the rate found by Natsis et alReference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 among Greek individuals (17%). Presence of a similar incidence of variation in two closely located populations such as Turks and Greeks may suggest a relationship between the incidence of aortic arch variations and ethnicity.
The number of studies investigating the relationship between the incidence of aortic arch variations and gender differences are limited. In 1976, MolzReference Molz 28 reviewed a series of 431 patients with an aberrant right subclavian artery in the literature and found that the occurrence of an aberrant subclavian artery was higher among females compared with males (59% versus 41%). Similarly, in a computed tomographic angiography study by Piyavisetpat et al,Reference Piyavisetpat, Thaksinawisut and Tumkosit 27 the incidence of an aberrant right subclavian artery was found to be higher among females compared with males (2.1% and 0.3%, respectively). In this study, it was found that the incidence of a left vertebral artery arising from the aortic arch was higher among males (5.8%) compared with females (2.1%), whereas the incidences of other variations of the aortic arch were similar in both genders. In a study by Natsis et al,Reference Natsis, Tsitouridis, Didagelos, Fillipidis, Vlasis and Tsikaras 15 the incidence of a “bovine” aortic arch was higher among males compared with females (69.8% versus 30.2%). In our study, we observed that the total incidence of variations of aortic arch branching was similar among males and females (20% versus 22.1%). In our study, the incidence of an aberrant right subclavian artery – combination of type 5 and type 6 variations – was higher among females compared with males (2.5% versus 0.5), which is similar to what has been reported in previous studies.
In conclusion, to the best of our knowledge, this study is one of the largest studies analysing the variations of the aortic arch branching pattern in a living patient population. The results of this study pointed out that variations in aortic arch branching are commonly seen in the Turkish population. Recognition of the variations of the aortic arch branching pattern is important because they may cause symptoms because of tracheoesophageal compression or complications during surgical or endovascular interventional procedures of the aorta and its branches. Computed tomographic angiography is a non-invasive imaging modality that should be preferred in detailed visualisation of the supra-aortic vascular anatomy because of its high spatial resolution and multi-planar and three-dimensional imaging capabilities.
Conflicts of Interest
None.










