Published online by Cambridge University Press: 24 May 2005
Objective: To document the echocardiographic features of tetralogy of Fallot during fetal and postnatal life. Correlation of echocardiographic findings with the requirement for early intervention prior to definitive repair. Design: Retrospective observational study. Setting: A tertiary fetal cardiology unit. Patients: Fetuses with a diagnosis of tetralogy of Fallot identified from a prospective database between 1 January 1999 and 31 October 2002. Main measures of outcome: Growth of aorta and pulmonary trunk during fetal and postnatal life. Doppler assessment of the great arteries both prenatally and postnatally. Clinical outcome to definitive repair. Results: We identified 25 fetuses with tetralogy of Fallot, 23 having a pulmonary valvar diameter below the normal range at some point during gestation. The ratio of the diameter of the aortic to the pulmonary valve was abnormal in all cases. The pulmonary arterial Doppler velocity was within the normal range in six fetuses at presentation, and elevated in the remainder. In two fetuses, the right ventricular outflow tract was patent during fetal life, but had become atretic at birth. Both of these fetuses had reversal of flow in the arterial duct at presentation during fetal life. In 2 fetuses in whom we showed poor growth of the pulmonary trunk in late gestation, it was necessary to intervene early. The Doppler velocity across the pulmonary valve during fetal life did not differentiate between babies who required early intervention and those who were repaired electively. There was a marked increase in pulmonary arterial Doppler velocity following birth, which became more elevated with age. Of 18 liveborn infants, 17 have survived, with 2 having balloon dilation of the right ventricular outflow tract, and 3 insertion of a Blalock-Taussig shunt prior to definitive repair. Conclusions: In tetralogy of Fallot, features of pulmonary valvar hypoplasia and obstruction are evident during fetal life. Progression of obstruction in the right ventricular outflow tract was observed during fetal life as well as postnatally. Reversal of flow in the arterial duct, and failure of growth of the pulmonary trunk, predicted the need for early surgery to maintain pulmonary blood flow. Parents should be counselled about the possibility of emergency intervention being required after birth. Affected fetuses should be delivered at units with experience of managing the cyanosed newborn.
The anatomy of tetralogy of Fallot as seen in infants and children has been well described, with a reported incidence of 2–2.6 per 10,000 live births.1, 2 There is a spectrum of obstruction in the right ventricular outflow tract, which characteristically progresses with age. It is recognised that the clinical symptoms, and hence requirements for intervention, depend on the degree of obstruction in the right ventricular outflow tract. Severe obstruction to pulmonary blood flow is associated with duct-dependent pulmonary circulation, necessitating palliation soon after birth. Fetal echocardiography allows identification of congenital cardiac disease, including tetralogy of Fallot. There is little data on the prenatal patterns of growth and flow in the great arteries in patients with tetralogy of Fallot.3–5
The aim of this longitudinal observational study was to document the antenatal and postnatal echocardiographic features of tetralogy of Fallot, with emphasis on growth of the pulmonary and aortic valves, and Doppler patterns of flow across them. In addition, we wished to observe whether the antenatal and postnatal sonographic findings correlated with the timing of surgical correction or palliation.
We included fetuses with tetralogy of Fallot assessed at our tertiary fetal cardiology unit between 1 January 1999 and 31 October 2002. Cases were selected using our prospective database (Filemaker Pro V 4.0, Claris Corporation, Santa Clara, CA, USA). During this period, a total of 25 fetuses were diagnosed with tetralogy of Fallot. The gestational age at examination was determined by obstetric ultrasonic scanning in all cases. Infants were followed until either elective correction or earlier intervention. The last measurements included were those immediately prior to surgery or catheter intervention.
Fetal echocardiograms were all performed using an Agilent 5500 ultrasound system (Agilent Inc. Andover, Mass., USA). Cross-sectional echocardiography was used to measure the internal diameters of the aorta and pulmonary trunk at the level of the arterial valves at end-diastole. Pulsed Doppler velocities across the aortic and pulmonary valves were recorded with angle correction not exceeding 15 degrees. Where possible, measurements were made with the ultrasonic beam perpendicular to the vessel wall, but this was not always possible due to fetal position. To minimize observer error, videotapes of all the fetal echocardiograms were analysed by a single observer (JMS). Ideally, we would have preferred to measure the minimum diameter of the subpulmonary area, but this did not prove possible retrospectively.
Postnatal echocardiography was performed using Vingmed CFM 800, General Electric Vivid 3 (General Electric Corporation, Milwaukee, USA), Agilent 2000 or 5500 ultrasound systems (Agilent Inc, Andover, Mass, USA) with 3–12 MHz transducers, measuring the pulmonary and aortic valvar diameters. In most cases, only flow across the pulmonary valve was interrogated by Doppler, because flow was laminar across the aortic valve. The pulmonary valvar diameter was determined from the short axis view of the heart, at end-diastole, at the level of the valve. Aortic valvar diameter was determined from the long axis view of the left ventricular outflow tract. Measurements were made of the internal diameter of the vessel at the level of insertion of the valvar leaflets at end-diastole. Doppler velocity at the level of the pulmonary valve was measured from the short axis view at an insonation angle to the flow of less than 15°. At least five consecutive uniform Doppler velocity waveforms were observed and recorded. Peak velocities were then averaged.
Peak Doppler velocities across the right ventricular outflow tract were documented. The diameters of the arterial valves, and corresponding ratios, were measured. Data points were compared to graphs of normal values. The studies reporting these normal ranges were selected to ensure that the data was recorded in a similar manner. The normal reference ranges used were: fetal pulmonary and aortic valvar diameters;6 ratio of aortic to pulmonary fetal valvar diameters;6 fetal pulmonary valvar Doppler velocities;7 fetal aortic valvar Doppler velocities;8 ratio of velocities across the aortic and pulmonary valves;6, 8 normal pulmonary valvar and aortic diameters in neonates and infants;9 normal ranges of Doppler velocities across the pulmonary valve in neonates and infants.10
Infants born under our care were discharged only after reassurance that their saturations of oxygen in air were greater than 80%, and serial echocardiograms had verified that either their duct had closed, or was not thought to provide significant pulmonary blood flow. The policy in our hospital is electively to repair infants with tetralogy of Fallot at the age of 6 to 12 months when weighing in excess of 6 kilograms. For symptomatic infants, those having excessive or inadequate flow of blood to the lungs, correction may be considered earlier. The option of a temporizing intervention, by means of contruction of a Blalock-Taussig shunt or balloon dilation of the right ventricular outflow tract, is also considered. Serial echocardiograms were performed until the infants reached the appropriate weight for elective correction, or until an early intervention was required. In this study, early intervention was considered to be “early” correction, or a temporizing procedure as described above, thus separating the patients into two groups, those having elective correction or requiring early intervention.
Pregnancies that ended in termination were excluded from analysis. Fetuses with pulmonary atresia at initial presentation were excluded from analysis because all will have retrograde flow of blood from the arterial duct or from aortopulmonary collateral arteries. In addition, fetuses with tetralogy of Fallot and “absent pulmonary valve” were also excluded, because their echocardiographic features and postnatal management is different from “classical” tetralogy of Fallot.11 We also excluded fetuses that had an atrioventricular septal defect in association with tetralogy of Fallot.
We identified 25 fetuses with tetralogy of Fallot during the chosen period. In five cases, the parents elected to terminate the pregnancy. There were chromosomal abnormalities in two of these, one each with trisomy 21 and trisomy 18. The details of the 20 continuing pregnancies are shown in Table 1. Of the 20 continuing pregnancies, 15 had been referred because of abnormal routine obstetric scans. In two cases, congenital cardiac disease had been found in a previous pregnancy, one with ventricular septal defect and the other with discordant ventriculo-arterial connections, ventricular septal defect, and pulmonary stenosis. Another case had paternal history of congenital cardiac disease, specifically a small ventricular septal defect, and the final two mothers were referred because of increased nuchal translucency. Of the 18 infants who have delivered, 12 were born at our center, and the remaining six in other hospitals. Of these six, two were referred to us, and are included in our postnatal echocardiographic data. The remaining four are followed up at other cardiac centers. Their postnatal echocardiograms were not available for review.
Table 1. Characteristics of fetuses with tetralogy of Fallot.

In total, 44 echocardiograms were assessed, at a gestational age ranging from 15 through 38 weeks. The fetal echocardiographic data is shown in Figures 1–6. In all but two cases, the pulmonary valvar diameter was below the normal range at some point during gestation (Fig. 1). The aortic valvar diameters tended to increase across centiles with advancing gestation (Fig. 2), and the ratio of aortic to pulmonary valvar diameter was abnormal in all fetuses (Fig. 3). The aortic valvar Doppler velocity was normal (Fig. 4). The Doppler velocity across the pulmonary valve was above the normal range in all cases at some stage in pregnancy, with some overlap with the upper half of the normal range (Fig. 5). The ratio of aortic to pulmonary Doppler velocity was abnormal in all fetuses at some point in gestation (Fig. 6). In two fetuses, cases 14 and 18, there was reversal of flow in the arterial duct at presentation during fetal life.
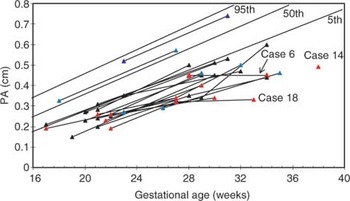
Figure 1. Graph of pulmonary arterial diameters plotted against gestational age. Normal centiles are indicated by blue lines. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention. The three fetuses who required Blalock Taussig shunts soon after birth are indicated.
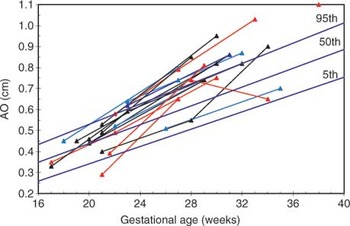
Figure 2. Graph of fetal aortic diameters plotted against gestational age. Normal centiles are indicated by solid lines. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention.

Figure 3. Ratio of aortic to pulmonary arterial dimension in fetuses with tetralogy of Fallot. Normal centiles are indicated by solid blue lines. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention.

Figure 4. Aortic Doppler velocity in fetuses with tetralogy of Fallot. Normal centiles are indicated by solid lines. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention.

Figure 5. Pulmonary arterial Doppler velocity in fetuses with tetralogy of Fallot. Normal centiles are indicated by solid blue lines. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention.

Figure 6. Ratio of aortic to pulmonary arterial Doppler velocity in fetuses with tetralogy of Fallot. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention.
The pulmonary valvar diameter was below the 50th centile in all but one case (Fig. 7). The pulmonary valvar diameters of those infants requiring early intervention appeared slightly lower than those repaired electively, when plotted against body weight, but there was considerable overlap (Fig. 7). The aortic valvar diameter was above the 50th centile in all but one case (Fig. 8). This resulted in abnormal ratios of the diameters of the aortic and pulmonary valves (Fig. 9). The pulmonary arterial Doppler velocity was elevated, and increased with postnatal age and weight (Figs 10 and 11).

Figure 7. Pulmonary valvar diameter plotted against weight for infants with tetralogy of Fallot. Normal centiles are indicated by solid blue lines. Black data points – elective correction, red data points – early intervention, blue data points less than 4 months and has not required intervention yet. The two fetuses who developed pulmonary atresia are indicated by arrows.

Figure 8. Aortic diameter plotted against weight for infants with tetralogy of Fallot. Normal centiles are indicated by solid lines. Black data points – elective correction, red data points – early intervention, blue data points less than 4 months and has not required intervention yet.

Figure 9. Ratio of aortic to pulmonary valvar diameter for infants with tetralogy of Fallot. Normal centiles are indicated by solid lines. Black data points – elective correction, red data points – early intervention, blue data points less than 4 months and has not required intervention yet.
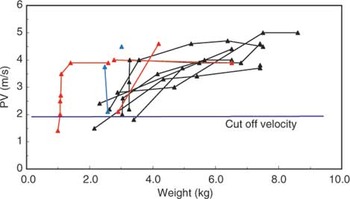
Figure 10. Pulmonary arterial Doppler velocity plotted against weight for infants with tetralogy of Fallot. Black data points – elective correction, red data points – early intervention, blue data points less than 4 months and has not required intervention yet.
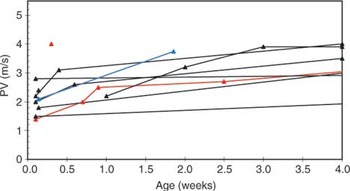
Figure 11. Pulmonary arterial Doppler velocity plotted against postnatal age for infants with tetralogy of Fallot. Black data points – elective correction, red data points – early intervention, blue data points less than 4 months and has not required intervention yet.
The clinical outcome is shown as a flowchart in Figure 12. The median gestational age at delivery was 37.9 weeks, ranging from 32 to 40.6 weeks. Three infants were born prior to term, one at 32 weeks and two at 35 weeks. One of the infants, Case 6, red triangle in figures, who was born at a district general hospital, was transferred on the first day of life to another cardiac centre for emergency construction of a modified Blalock-Taussig shunt because of severe desaturations. In two fetuses, cases 14 and 18, forward flow of blood was documented across the pulmonary valve in the second trimester, but pulmonary atresia had developed by birth. Of these cases, one was referred to our center at 38 weeks gestation from another cardiac centre and had forward flow documented across the pulmonary valve at 1.6 metres per second, although reversal of flow in the arterial duct was also noted. In the other case, case 18, a small jet of forward flow of blood was seen on colour flow Doppler in the mid trimester, but none on the final fetal echocardiogram. Reversal of flow in the arterial duct was noted throughout. It was not possible to align the Doppler reliably to the colour jet in this case. Both of these fetuses had a right-sided Blalock-Taussig shunt inserted in the first week following delivery. In two infants, cases 10 and 11, saturations were sufficient for them to be discharged home initially, but they represented with low oxygen saturations. Both underwent balloon dilation of the right ventricular outflow tract at 4 months of age, and underwent successful elective repair later in infancy.
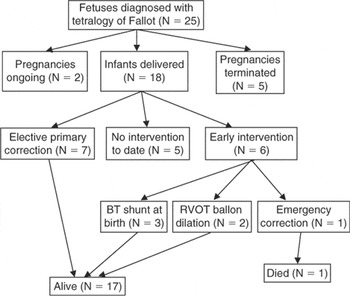
Figure 12. Flowchart illustrating the outcome of the twelve fetuses diagnosed with tetralogy of Fallot.
Overall, 17 of the 18 live-born infants survived. The baby who died, our first case, had a large fistula from the left coronary artery draining to the left atrial appendage. This baby was small for dates, with a weight at birth of 990 g. The postnatal course was complicated by volume loading of the left ventricle due to the fistula and significant desaturations from 14 weeks onwards, when the baby weighed 2.5 kg. The option of a Blalock-Taussig shunt was rejected because this would have increased the volume load on the left ventricle. Correction was therefore undertaken, coupled with surgical ligation of the coronary arterial fistula. The baby initially made good postoperative progress, and was extubated, but died 5 days postoperatively secondary to poor left ventricular function.
Of the remaining infants, 7 were repaired electively. The median age at time of surgery was 7.9 months. The oldest age at surgery was 15.5 months in a child with multiple extracardiac abnormalities and poor growth. The youngest age of elective surgery was 6.3 months. The median body weight at time of elective correction was 7.4 kg, ranging from 6.5 kg to 8.6 kg. One infant, who was under the care of another hospital, had a modified Blalock-Taussig shunt inserted at 8 months of age. This reflected a difference of policy for repair of tetralogy of Fallot compared to our institution. We were uncertain how to classify this baby for the purpose of comparison of the fetuses requiring early versus late intervention. We have included this infant as an elective “correction”, given that primary correction would probably have been favoured at our hospital.
As far as we are aware, this is the first study of tetralogy of Fallot to document serial changes in echocardiographic parameters throughout fetal and postnatal life until intervention. Unlike previous series.3, 4 we excluded cases of tetralogy of Fallot with pulmonary atresia at presentation. We did include two fetuses who acquired pulmonary atresia with advancing gestational age. Hornberger et al.3 reported the postnatal outcomes of sixteen fetuses diagnosed with tetralogy of Fallot, two of whom had pulmonary atresia. The remaining fourteen had similar pulmonary and aortic valvar diameters and trends of growth compared to our series. Hornberger et al.3 found that Doppler velocities across the pulmonary valve were normal or mildly elevated. Our data showed that, at some point during gestation, all of our fetuses had a Doppler velocity above the 50th centile, and most had a Doppler velocity above the normal range at some point, although the degree of elevation was not marked (Fig. 5). Thus, although the right ventricle faces a high pulmonary vascular resistance during fetal life, the Doppler velocity is usually increased, although not to a major degree.
Another study, by Lee et al.,4 reported the postnatal outcome of 17 cases of tetralogy of Fallot diagnosed antenatally. The pulmonary valvar diameters we found were also very similar to that series, although the normal ranges were different, and did not appear to be modeled to take account of increasing gestational age.12 The ratio of aortic to pulmonary valvar diameters was always abnormal in our series. This finding is important, because views of the outflow tracts are obtained during routine anomaly scans, and any major discrepancy in the size of the great arteries merits detailed specialist fetal echocardiography.
One of the aims of our study was to predict which fetuses require early intervention. Both of the fetuses with reversal of flow in the arterial duct went on to develop acquired pulmonary atresia and required shunting in the neonatal period. Of those fetuses who had antegrade flow across the pulmonary valve throughout gestation, it was difficult to distinguish the group who would require early intervention from those who would not, although two cases did demonstrate growth failure of the pulmonary trunk. Interestingly, when the pulmonary valvar diameter measured postnatally was plotted against body weight, those fetuses requiring early intervention did appear to have lower values than those who were repaired electively, and similarly the ratio of aorta to pulmonary arterial size was higher when plotted against body weight. We made no attempt to measure fetal weight, and cannot obtain this information retrospectively, but this may be relevant for prospective work.
Azancot et al.5 reported a series of 44 cases of prenatally diagnosed tetralogy of Fallot, and subdivided cases into those with major extracardiac abnormalities and those with “isolated” tetralogy of Fallot. The survival in the two groups was 10% and 84%, respectively, confirming the profound influence of extracardiac malformations on prognosis. Azancot et al.5 did not present detailed postnatal information, but did observe a relationship between the pulmonary arterial size and the ratio of the aorta to the pulmonary trunk with the timing of surgery. This contrasts with the current study.
Again, to the best of our knowledge, there are no previous series that include detailed postnatal echocardiographic information of fetuses with tetralogy of Fallot diagnosed during fetal life. Postnatally, pulmonary valvar diameters showed either low or normal values. There was some tendency for reduced growth with advancing postnatal age compared to normal. The aortic valvar diameters tended to increase relative to centiles with advancing postnatal age. The Doppler velocity across the pulmonary valve increased with postnatal age and weight. The acute change in Doppler velocity from prenatal to postnatal life reflects the initial decrease in pulmonary vascular resistance, permitting a larger flow across the pulmonary valve. In the longer term, increasing obstruction of the right ventricular outflow tract will also contribute to this trend of elevated Doppler velocities across the pulmonary valve.
In three babies, it proved necessary to construct Blalock-Taussig shunts in the first week postnatally (cases 6, 14,18). Cases 6 and 18 were studied sequentially, and had subnormal growth of the pulmonary trunk, particularly late in gestation (Fig. 1). One of these fetuses developed pulmonary atresia prenatally (case 18), but the other (case 6) had antegrade flow across the pulmonary valve, with no reversal of ductal flow, on the final fetal echocardiogram at 34 weeks. Thus, failure of growth of the pulmonary trunk may be helpful in predicting the need for early postnatal intervention, and fetal echocardiography at a relatively late stage in gestation appears warranted. Two of these fetuses (cases 14 and 18) demonstrated reversal of flow in the arterial duct, which was not observed in any other fetuses in this series. Firm predictors of early intervention, nonetheless, will require to be established in a larger prospective series. When parents are counseled regarding postnatal management, they should be alerted that emergency intervention is occasionally required. Prenatal identification of these infants is difficult. Thus, such infants should be delivered at units that have adequate facilities to manage the cyanosed newborn.
The majority of infants in our series underwent elective correction, with 17 of 18 liveborn infants surviving. A previous study documented a high mortality (75%) following prenatal diagnosis of tetralogy of Fallot.13 The mortality was mainly due to extracardiac and chromosomal abnormalities. Significant extracardiac abnormalities were present in five of our patients, but despite this overall survival was good. This reflects improved surgical results, but referral patterns may also influence mortality. An increasing number of fetuses with major anomalies are detected by use of early screening tests such as nuchal translucency, serum screening, or early anomaly scans. This may result in termination of pregnancy at an early stage, without fetal echocardiography. Two continuing pregnancies in the current study had chromosomal abnormalities, including a chromosome 22q11 deletion which has been increasingly recognized with this lesion,14 and an XXY karyotype which has not previously been linked to tetralogy of Fallot. The one baby who died was a small infant with an associated large coronary arterial fistula. In this case, left ventricular dysfunction was the cause of death, rather than the usual pattern of malformations associated with tetralogy of Fallot.
The relatively small size of our sample makes detailed statistical analysis inappropriate. The timing of prenatal and postnatal scans was not standardized. Ideally, relevant measurements would have been made prospectively. We attempted to minimize observer error by using a single observer during fetal life, and two observers for postnatal analysis. Three sets of postnatal echocardiographic data were not available to us for analysis. We would have preferred to assess the diameter and Doppler velocity of flow through the subpulmonary area as well as the pulmonary valve but this did not prove possible retrospectively.
We would like to thank Dr Michael Burch for postnatal data on one patient managed at the John Radcliffe Hospital, Oxford, and Dr Alan Magee for postnatal information on one patient managed at the Royal Brompton Hospital. Dr Nigel Smeeton is thanked for statistical advice.

Table 1.

Graph of pulmonary arterial diameters plotted against gestational age. Normal centiles are indicated by blue lines. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention. The three fetuses who required Blalock Taussig shunts soon after birth are indicated.

Graph of fetal aortic diameters plotted against gestational age. Normal centiles are indicated by solid lines. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention.

Ratio of aortic to pulmonary arterial dimension in fetuses with tetralogy of Fallot. Normal centiles are indicated by solid blue lines. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention.

Aortic Doppler velocity in fetuses with tetralogy of Fallot. Normal centiles are indicated by solid lines. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention.

Pulmonary arterial Doppler velocity in fetuses with tetralogy of Fallot. Normal centiles are indicated by solid blue lines. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention.

Ratio of aortic to pulmonary arterial Doppler velocity in fetuses with tetralogy of Fallot. Black data points – elective correction, red data points – early intervention, blue data points ongoing pregnancy or infant not yet reached 4 months of age but has not needed intervention.

Pulmonary valvar diameter plotted against weight for infants with tetralogy of Fallot. Normal centiles are indicated by solid blue lines. Black data points – elective correction, red data points – early intervention, blue data points less than 4 months and has not required intervention yet. The two fetuses who developed pulmonary atresia are indicated by arrows.

Aortic diameter plotted against weight for infants with tetralogy of Fallot. Normal centiles are indicated by solid lines. Black data points – elective correction, red data points – early intervention, blue data points less than 4 months and has not required intervention yet.

Ratio of aortic to pulmonary valvar diameter for infants with tetralogy of Fallot. Normal centiles are indicated by solid lines. Black data points – elective correction, red data points – early intervention, blue data points less than 4 months and has not required intervention yet.

Pulmonary arterial Doppler velocity plotted against weight for infants with tetralogy of Fallot. Black data points – elective correction, red data points – early intervention, blue data points less than 4 months and has not required intervention yet.

Pulmonary arterial Doppler velocity plotted against postnatal age for infants with tetralogy of Fallot. Black data points – elective correction, red data points – early intervention, blue data points less than 4 months and has not required intervention yet.

Flowchart illustrating the outcome of the twelve fetuses diagnosed with tetralogy of Fallot.